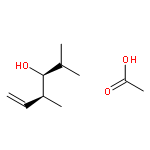
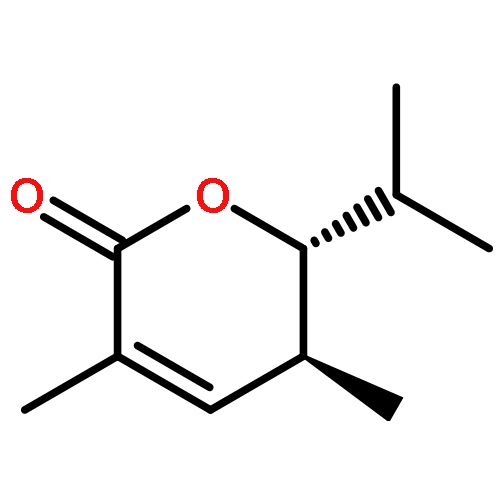
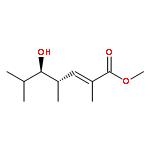
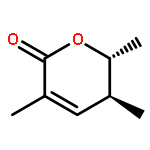
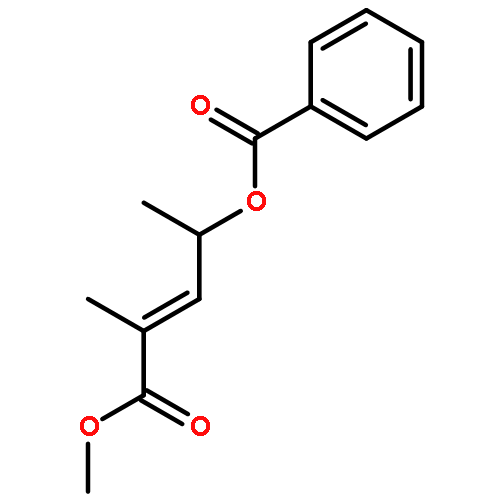
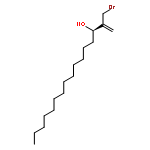
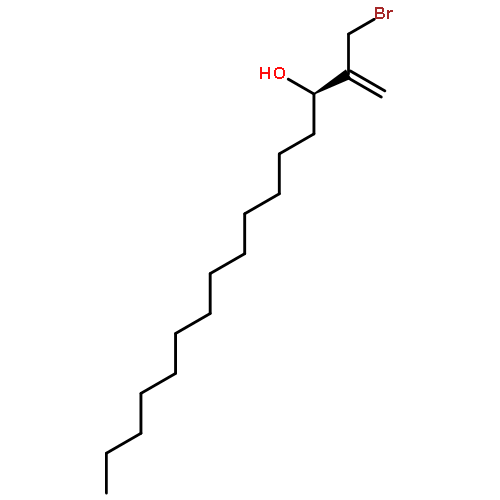
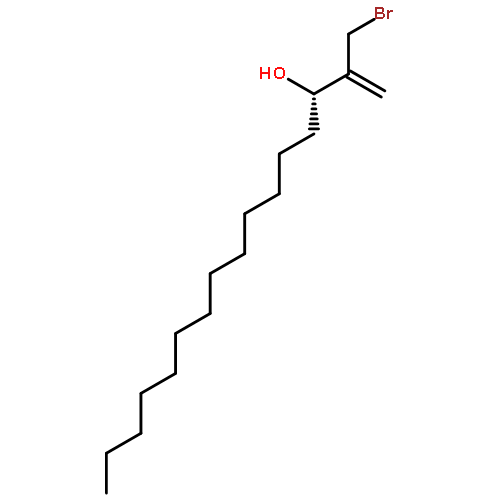
Co-reporter:Frank E. McDonald, Kento Ishida, Jessica A. Hurtak
Tetrahedron 2013 69(36) pp: 7746-7758
Publication Date(Web):
DOI:10.1016/j.tet.2013.04.138
Co-reporter:Matthew A. Boone ; Rongbiao Tong ; Frank E. McDonald ; Sheri Lense ; Rui Cao ;Kenneth I. Hardcastle
Journal of the American Chemical Society 2010 Volume 132(Issue 14) pp:5300-5308
Publication Date(Web):March 24, 2010
DOI:10.1021/ja1006806
We achieved the stereoselective syntheses of two different structural patterns corresponding to the enantiomers of the marine natural products abudinol B and muzitone, by developing two-directional tandem biomimetic cyclizations of polyepoxides of squalene analogues in which one alkene was functionalized as an enolsilane. In the course of this work, we demonstrated that the structure of muzitone was misassigned.
Co-reporter:Claney L. Pereira ; Yi-Hung Chen
Journal of the American Chemical Society 2009 Volume 131(Issue 17) pp:6066-6067
Publication Date(Web):April 8, 2009
DOI:10.1021/ja9009265
The first total synthesis of the sphingolipid biosynthesis inhibitor fumonisin B1 has been achieved. This convergent synthesis utilizes oxonia Cope rearrangements to prepare two key homoallylic alcohols, which are then functionalized to the primary components A and B for cross-coupling. Other highlights of our approach include a new and efficient synthesis of the diprotected tricarballylic acid C and a global deprotection strategy as the final step.
Co-reporter:Bradley R. Balthaser and Frank E. McDonald
Organic Letters 2009 Volume 11(Issue 21) pp:4850-4853
Publication Date(Web):September 25, 2009
DOI:10.1021/ol901923x
An acid-promoted glycosylation and alkynol cycloisomerization sequence provided direct access to the 2-deoxytrisaccharide corresponding to the fucose−saccharosamine−digitoxose substructure of saccharomicin B. In the course of this work, the absolute stereochemistry of the repeating fucose−saccharosamine disaccharide of saccharomicins was also confirmed.
Co-reporter:Omar Robles and Frank E. McDonald
Organic Letters 2009 Volume 11(Issue 23) pp:5498-5501
Publication Date(Web):November 10, 2009
DOI:10.1021/ol902365n
A highly convergent synthesis of fostriecin is described, featuring sequential palladium-catalyzed Negishi cross couplings to form the C7−C8 bond and C8−methyl bond, followed by late-stage regio- and stereoselective dihydroxylation of C8−C9.
Co-reporter:Rongbiao Tong Dr. ;FrankE. McDonald Dr.
Angewandte Chemie International Edition 2008 Volume 47( Issue 23) pp:4377-4379
Publication Date(Web):
DOI:10.1002/anie.200800749
Co-reporter:Rongbiao Tong Dr. ;FrankE. McDonald Dr.
Angewandte Chemie 2008 Volume 120( Issue 23) pp:4449-4451
Publication Date(Web):
DOI:10.1002/ange.200800749
Co-reporter:Frank E McDonald, K.Subba Reddy
Journal of Organometallic Chemistry 2001 Volumes 617–618() pp:444-452
Publication Date(Web):15 January 2001
DOI:10.1016/S0022-328X(00)00643-4
An account of the discovery of the tungsten carbonyl-catalyzed endo-selective cycloisomerization of alkynyl alcohols to dihydropyrans is described. The utility of this new chemical transformation involving tungsten vinylidene catalytic intermediates is demonstrated in stereoselective syntheses of disaccharide substructures 26–28 of the landomycin and mithramycin families of anticancer antibiotics.
Co-reporter:Frank E. McDonald Dr.;K. Subba Reddy Dr.
Angewandte Chemie International Edition 2001 Volume 40(Issue 19) pp:
Publication Date(Web):2 OCT 2001
DOI:10.1002/1521-3773(20011001)40:19<3653::AID-ANIE3653>3.0.CO;2-W
Tungsten-catalyzed alkynol cycloisomerization and iterative acid-catalyzed stereoselective glycosylations form the basis of a revolutionary new strategy for oligosaccharide synthesis. The method has been successfully applied to a highly convergent synthesis of Digitalis 2-deoxyglycosides (see scheme).
Co-reporter:Frank E. McDonald Dr.;K. Subba Reddy Dr.
Angewandte Chemie 2001 Volume 113(Issue 19) pp:
Publication Date(Web):2 OCT 2001
DOI:10.1002/1521-3757(20011001)113:19<3765::AID-ANGE3765>3.0.CO;2-1
Wolfram-katalysierte Alkinolcycloisomerisierung und iterative säurekatalysierte stereoselektive Glycosylierungen bilden die Grundlage für eine völlig neue Strategie zur Oligosaccharidsynthese. Die Methode wurde bei der hochkonvergenten Synthese von Digitalis-2-Desoxyglycosiden erfolgreich angewendet (siehe Schema).
Co-reporter:Frank E. McDonald
Chemistry - A European Journal 1999 Volume 5(Issue 11) pp:
Publication Date(Web):29 OCT 1999
DOI:10.1002/(SICI)1521-3765(19991105)5:11<3103::AID-CHEM3103>3.0.CO;2-N
The discovery of new chemical transformations permits exploration and development of novel synthesis strategies. This account describes the invention of endo-selective cycloisomerizations of alkynyl alcohols, and mechanistically related transformations of terminal alkynes tethered to nitrogen, carbon, and sulfur nucleophiles (see diagram). The synthetic utility of these new transformations is exemplified in applications to anti-AIDS nucleosides, polycyclic ethers, and oligosaccharides.