Co-reporter:Maria M. Shchepinova, Andrew G. Cairns, Tracy A. Prime, Angela Logan, ... Richard C. Hartley
Cell Chemical Biology 2017 Volume 24, Issue 10(Volume 24, Issue 10) pp:
Publication Date(Web):19 October 2017
DOI:10.1016/j.chembiol.2017.08.003
•MitoNeoD is a mitochondria-targeted O2⋅− probe that can be used in vivo•Neopentyl groups prevent DNA intercalation by MitoNeoD and its derivatives•Incorporation of a carbon-deuterium bond enhances O2⋅− selectivity by MitoNeoD•MitoNeoD extends methods available to assess mitochondrial O2⋅−Mitochondrial superoxide (O2⋅−) underlies much oxidative damage and redox signaling. Fluorescent probes can detect O2⋅−, but are of limited applicability in vivo, while in cells their usefulness is constrained by side reactions and DNA intercalation. To overcome these limitations, we developed a dual-purpose mitochondrial O2⋅− probe, MitoNeoD, which can assess O2⋅− changes in vivo by mass spectrometry and in vitro by fluorescence. MitoNeoD comprises a O2⋅−-sensitive reduced phenanthridinium moiety modified to prevent DNA intercalation, as well as a carbon-deuterium bond to enhance its selectivity for O2⋅− over non-specific oxidation, and a triphenylphosphonium lipophilic cation moiety leading to the rapid accumulation within mitochondria. We demonstrated that MitoNeoD was a versatile and robust probe to assess changes in mitochondrial O2⋅− from isolated mitochondria to animal models, thus offering a way to examine the many roles of mitochondrial O2⋅− production in health and disease.Download high-res image (175KB)Download full-size image
Co-reporter:Stuart T. Caldwell, Andrew G. Cairns, Marnie Olson, Susan Chalmers, Mairi Sandison, William Mullen, John G. McCarron, Richard C. Hartley
Tetrahedron 2015 Volume 71(Issue 51) pp:9571-9578
Publication Date(Web):23 December 2015
DOI:10.1016/j.tet.2015.10.052
Changes in high localised concentrations of Ca2+ ions are fundamental to cell signalling. The synthesis of a dual excitation, ratiometric calcium ion sensor with a Kd of 90 μM, is described. It is tagged with an azido group for bioconjugation, and absorbs in the blue/green and emits in the red region of the visible spectrum with a large Stokes shift. The binding modulating nitro group is introduced to the BAPTA core prior to construction of a benzofuran-2-yl carboxaldehyde by an allylation–oxidation–cyclisation sequence, which is followed by condensation with an azido-tagged thiohydantoin. The thiohydantoin unit has to be protected with an acetoxymethyl (AM) caging group to allow CuAAC click reaction and incorporation of the KDEL peptide endoplasmic reticulum (ER) retention sequence.Figure optionsDownload full-size imageDownload as PowerPoint slide
Co-reporter:Andrew G. Cairns;Dr. Hans Martin Senn;Dr. Michael P. Murphy;Dr. Richard C. Hartley
Chemistry - A European Journal 2014 Volume 20( Issue 13) pp:3742-3751
Publication Date(Web):
DOI:10.1002/chem.201304241
Abstract
5,6-Disubstituted phenanthridinium cations have a range of redox, fluorescence and biological properties. Some properties rely on phenanthridiniums intercalating into DNA, but the use of these cations as exomarkers for the reactive oxygen species (ROS), superoxide, and as inhibitors of acetylcholine esterase (AChE) do not require intercalation. A versatile modular synthesis of 5,6-disubstituted phenanthridiniums that introduces diversity by Suzuki–Miyaura coupling, imine formation and microwave-assisted cyclisation is presented. Computational modelling at the density functional theory (DFT) level reveals that the novel displacement of the aryl halide by an acyclic N-alkylimine proceeds by an SNAr mechanism rather than electrocyclisation. It is found that the displacement of halide is concerted and there is no stable Meisenheimer intermediate, provided the calculations consistently use a polarisable solvent model and a diffuse basis set.
Co-reporter:Stephen J. McQuaker;Dr. Casey L. Quinlan;Dr. Stuart T. Caldwell; Martin D. Br;Dr. Richard C. Hartley
ChemBioChem 2013 Volume 14( Issue 8) pp:993-1000
Publication Date(Web):
DOI:10.1002/cbic.201300115
Abstract
A high membrane potential across the mitochondrial inner membrane leads to the production of the reactive oxygen species (ROS) implicated in aging and age-related diseases. A prototypical drug for the correction of this type of mitochondrial dysfunction is presented. MitoDNP-SUM accumulates in mitochondria in response to the membrane potential due to its mitochondria-targeting alkyltriphenylphosphonium (TPP) cation and is uncaged by endogenous hydrogen peroxide to release the mitochondrial uncoupler, 2,4-dinitrophenol (DNP). DNP is known to reduce the high membrane potential responsible for the production of ROS. The approach potentially represents a general method for the delivery of drugs to the mitochondrial matrix through mitochondria targeting and H2O2-induced uncaging.
Co-reporter:Stephen J. McQuaker;Dr. Casey L. Quinlan;Dr. Stuart T. Caldwell; Martin D. Br;Dr. Richard C. Hartley
ChemBioChem 2013 Volume 14( Issue 8) pp:
Publication Date(Web):
DOI:10.1002/cbic.201390025
Co-reporter:Susan Chalmers ; Stuart T. Caldwell ; Caroline Quin ; Tracy A. Prime ; Andrew M. James ; Andrew G. Cairns ; Michael P. Murphy ; John G. McCarron
Journal of the American Chemical Society 2011 Volume 134(Issue 2) pp:758-761
Publication Date(Web):December 22, 2011
DOI:10.1021/ja2077922
Depolarization of an individual mitochondrion or small clusters of mitochondria within cells has been achieved using a photoactivatable probe. The probe is targeted to the matrix of the mitochondrion by an alkyltriphenylphosphonium lipophilic cation and releases the protonophore 2,4-dinitrophenol locally in predetermined regions in response to directed irradiation with UV light via a local photolysis system. This also provides a proof of principle for the general temporally and spatially controlled release of bioactive molecules, pharmacophores, or toxins to mitochondria with tissue, cell, or mitochondrion specificity.
Co-reporter:Marek Figlus, Natalie Wellaway, Anthony W. J. Cooper, Steven L. Sollis, and Richard C. Hartley
ACS Combinatorial Science 2011 Volume 13(Issue 3) pp:280
Publication Date(Web):March 25, 2011
DOI:10.1021/co100091n
Triphenylphosphine tagged with a short poly(ethyleneglycol)-ω-monomethyl ether chain (light MPEG, 10−16 ethylenoxy units, MTPP-G2) and an MPEG-tagged version of diethyl azodicarboxylate (MDEAD) have been used to prepare a 20 member library of esters, ethers, and sulfonamides, with cLogP’s in the range of 1.4−5.7 on a 0.1 mmol scale. Removal of MPEG-tagged side products was achieved by MPEG-assisted solid-phase extraction (MSPE) on prepacked silica columns to give the products in good yield and high purity.Keywords: cLogP; Mitsunobu reaction; solid-phase extraction; triphenylphosphine
Co-reporter:Adam Haahr, Zoran Rankovic, Richard C. Hartley
Tetrahedron Letters 2011 Volume 52(Issue 23) pp:3020-3022
Publication Date(Web):8 June 2011
DOI:10.1016/j.tetlet.2011.04.017
The combination of the Nysted reagent and titanocene dichloride methylenates aldehydes and ketones to give alkenes, and in a microwave-assisted process, esters and lactones give enol ethers. The methylenating agent in this one-pot procedure is presumed to be titanocene methylidene, which is the same reactive intermediate as that generated from Tebbe, Petasis and Grubbs reagents, each of which have to be prepared before use.

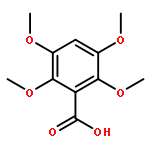

![2-Butanone, 1-(4'-nitro[1,1'-biphenyl]-4-yl)-4-phenyl-](http://img.cochemist.com/ccimg/620600/620595-05-3.png)
![2-Butanone, 1-(4'-nitro[1,1'-biphenyl]-4-yl)-4-phenyl-](http://img.cochemist.com/ccimg/620600/620595-05-3_b.png)
![1,3-Dioxolo[4,5-g]quinoline, 6-(3-furanyl)-](http://img.cochemist.com/ccimg/816500/816419-96-2.png)
![1,3-Dioxolo[4,5-g]quinoline, 6-(3-furanyl)-](http://img.cochemist.com/ccimg/816500/816419-96-2_b.png)
![Benzene, 1-methoxy-3-[(1E)-2-methoxyethenyl]-2-nitro-](http://img.cochemist.com/ccimg/816500/816419-83-7.png)
![Benzene, 1-methoxy-3-[(1E)-2-methoxyethenyl]-2-nitro-](http://img.cochemist.com/ccimg/816500/816419-83-7_b.png)


![1,3-Benzodioxole, 5-[(1E)-2-methoxyethenyl]-6-nitro-](http://img.cochemist.com/ccimg/816500/816419-85-9.png)
![1,3-Benzodioxole, 5-[(1E)-2-methoxyethenyl]-6-nitro-](http://img.cochemist.com/ccimg/816500/816419-85-9_b.png)
![1,3-Dioxolo[4,5-g]quinoline, 6-(2-phenylethyl)-](http://img.cochemist.com/ccimg/816500/816419-94-0.png)
![1,3-Dioxolo[4,5-g]quinoline, 6-(2-phenylethyl)-](http://img.cochemist.com/ccimg/816500/816419-94-0_b.png)
![Benzo[b]thiophen-5-amine, N,N-dimethyl-2-(2-methyl-1-propenyl)-](http://img.cochemist.com/ccimg/765300/765292-97-5.png)
![Benzo[b]thiophen-5-amine, N,N-dimethyl-2-(2-methyl-1-propenyl)-](http://img.cochemist.com/ccimg/765300/765292-97-5_b.png)
![Methanone, [(2R)-2-(3-methoxyphenyl)-1-piperidinyl]phenyl-](http://img.cochemist.com/ccimg/920600/920512-84-1.png)
![Methanone, [(2R)-2-(3-methoxyphenyl)-1-piperidinyl]phenyl-](http://img.cochemist.com/ccimg/920600/920512-84-1_b.png)

