Co-reporter:Stephen A. Bradley, Brian J. Bresnan, Sylvia M. Draper, Niall W. A. Geraghty, Mark Jeffares, Thomas McCabe, T. Brian H. McMurry and John E. O'Brien
Organic & Biomolecular Chemistry 2011 vol. 9(Issue 8) pp:2959-2968
Publication Date(Web):07 Feb 2011
DOI:10.1039/C0OB01131B
A series of 6-alkenyl-3-phenylcyclohex-2-enones has been synthesised and the structures of the products obtained from them on irradiation have been determined. The 6-propenyl compounds afforded a tricyclic ‘parallel’ [2 + 2] cycloaddition product and a bicyclic enone resulting from hydrogen abstraction in the biradical intermediate. The 6-butenyl and 6-pentenyl analogues gave ‘crossed’ cycloaddition products only. Although the regiochemistry of these cycloaddition reactions cannot be explained in terms of the ‘rule of five’, it is compatible with the concept of ‘biradical conformation control’ which is based on a consideration of the energy and structure of the possible 1,4-biradical intermediates.
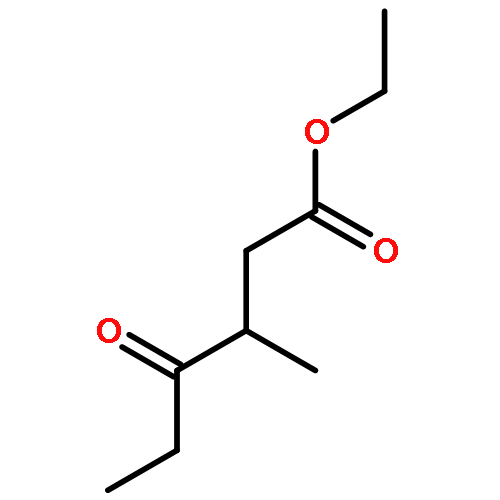
Co-reporter:Niall W.A. Geraghty, Patrick McArdle, Laverne M.A. Mullen
Tetrahedron 2011 67(19) pp: 3546-3552
Publication Date(Web):
DOI:10.1016/j.tet.2011.03.013
Co-reporter:Niall W.A. Geraghty, Elaine M. Hernon
Tetrahedron Letters 2009 50(5) pp: 570-573
Publication Date(Web):
DOI:10.1016/j.tetlet.2008.11.067
Co-reporter:Niall W. A. Geraghty and Andrea Lally
Chemical Communications 2006 (Issue 41) pp:4300-4302
Publication Date(Web):29 Aug 2006
DOI:10.1039/B608979H
The photomediated reaction of alkynes such as DMAD with 1,3-dioxolanes leads to remarkably rapid alkenylation; evidence is presented that these reactions occur via a chain mechanism.
Co-reporter:Roísín A. Doohan, John J. Hannan and Niall W. A. Geraghty
Organic & Biomolecular Chemistry 2006 vol. 4(Issue 5) pp:942-952
Publication Date(Web):06 Feb 2006
DOI:10.1039/B517631J
In the presence of a photomediator such as benzophenone, alkynes with electron-withdrawing groups react with cycloalkanes to give vinyl cycloalkanes. The reaction involves the regiospecific addition of a photochemically generated cycloalkyl radical to the β-carbon of the alkyne. The stereochemical outcome of the reaction depends on the nature of the photomediator and alkyne used.
Co-reporter:Roisin A. Doohan and Niall W. A. Geraghty
Green Chemistry 2005 vol. 7(Issue 2) pp:91-96
Publication Date(Web):10 Dec 2004
DOI:10.1039/B411786G
Using a standard mercury vapour lamp or sunlight, the synthetically difficult task of introducing functionality into unactivated cycloalkanes through C–C bond formation is accomplished in the presence of a soluble or supported photomediator and an alkyne bearing an electron-withdrawing group. The reaction involves the regiospecific addition of a photochemically generated cycloalkyl radical to the β-carbon of the alkyne. The use of solar radiation and a potentially recyclable polymer-bound photomediator for this fundamentally important synthetic process is particularly attractive from the clean/green chemistry perspective.
Co-reporter:Paul V. Murphy, Timothy J. O’Sullivan, Bryan D. Kennedy and Niall W. A. Geraghty
Organic & Biomolecular Chemistry 2000 (Issue 13) pp:2121-2126
Publication Date(Web):08 Jun 2000
DOI:10.1039/B001394N
Cyclic 2-diazo-1,3-dicarbonyl compounds react with diketene in the presence of rhodium(II) salts to give benzofurans as the major isolated products. The formation of intermediate products with exocyclic double bonds which isomerise to benzofurans provides support for the proposed mechanism which involves initial formation of a dioxaspirooctenone by a formal dipolar cycloaddition reaction of a carbenoid to the exocyclic double bond of diketene followed by the loss of carbon dioxide. Acyclic 2-diazo-1,3-dicarbonyl compounds give furans in poor yield.
Co-reporter:Paul V. Murphy, Timothy J. O’Sullivan and Niall W. A. Geraghty
Organic & Biomolecular Chemistry 2000 (Issue 13) pp:2109-2119
Publication Date(Web):08 Jun 2000
DOI:10.1039/B001393P
The cyclopropanespiro-β-lactones 3, 4 and 12 can be prepared by the metal catalysed, or photochemically promoted decomposition reactions of diazocompounds in the presence of diketene. The thermal reactions of these compounds give a variety of products depending on the nature of the spirolactone; these include a furan 9a, 1,4-dicarbonyl compounds 18a–c and 19b, a pyranone 20b, furanones 21a, 21f and 22a and the enol 16. The boron trifluoride promoted reaction of a mixture of 3b and 4b gives a β-ketoacid. Mechanisms are proposed for the formation of these products. The rearrangment of the cyclopropanespiro-β-lactones to furan-2(5H)-ones and furan-2-(3H)-ones 6–8, 21a, 21f, 22a and 24 is shown to be a general reaction that involves metal catalysis. A mechanism based on formation of a metallocycle by a novel insertion of the metal into the C–O bond of the β-lactone ring is proposed for this rearrangement. This accounts for the observed features of the reaction.
Co-reporter:Stephen A. Bradley, Brian J. Bresnan, Sylvia M. Draper, Niall W. A. Geraghty, Mark Jeffares, Thomas McCabe, T. Brian H. McMurry and John E. O'Brien
Organic & Biomolecular Chemistry 2011 - vol. 9(Issue 8) pp:NaN2968-2968
Publication Date(Web):2011/02/07
DOI:10.1039/C0OB01131B
A series of 6-alkenyl-3-phenylcyclohex-2-enones has been synthesised and the structures of the products obtained from them on irradiation have been determined. The 6-propenyl compounds afforded a tricyclic ‘parallel’ [2 + 2] cycloaddition product and a bicyclic enone resulting from hydrogen abstraction in the biradical intermediate. The 6-butenyl and 6-pentenyl analogues gave ‘crossed’ cycloaddition products only. Although the regiochemistry of these cycloaddition reactions cannot be explained in terms of the ‘rule of five’, it is compatible with the concept of ‘biradical conformation control’ which is based on a consideration of the energy and structure of the possible 1,4-biradical intermediates.
















![1,3-Dioxolane, 2-methyl-2-[(1E)-2-[(4-methylphenyl)sulfonyl]ethenyl]-](http://img.cochemist.com/ccimg/916300/916220-62-7.png)
![1,3-Dioxolane, 2-methyl-2-[(1E)-2-[(4-methylphenyl)sulfonyl]ethenyl]-](http://img.cochemist.com/ccimg/916300/916220-62-7_b.png)