Co-reporter:Zhen-Hua Liu, Rui-Jing Ma, Liu Yang, Jin-Yu Li, Bo Hou, Jiang-Miao Hu, Jun Zhou
Fitoterapia 2017 Volume 119(Volume 119) pp:
Publication Date(Web):1 June 2017
DOI:10.1016/j.fitote.2017.04.011
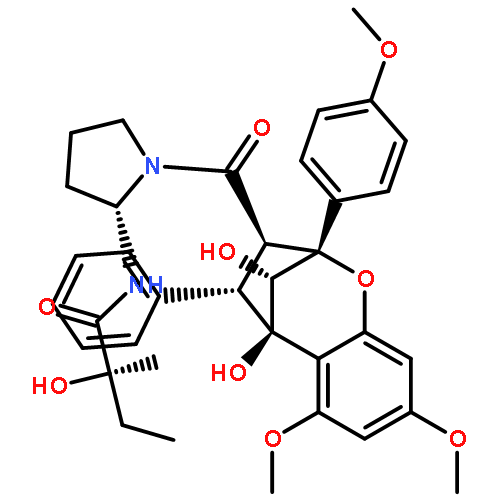
Three new triterpenoids, patrinolides B-D (1–3), and two new iridoids, patriscabioins K–L (9–10), together with five known compounds (4–8) were isolated from the extract of the whole plants of Patrinia scabiosaefolia. Compounds 1, 9, and 10 contained the unique substituents in Valerianaceae family, such as isovalery and 3-methylcrotonyl. Compound 2 was a 24-nor-ursane triterpenoid. Their structures were established on the basis of extensive spectroscopic analysis (UV, IR, MS, 1D and 2D NMR). The inhibitory activities against nitric oxide synthase (NOS) of all triterpenoids were tested. The results showed that compound 4 had moderate inhibitory activity with IC50 of 10.1 μM. Furthermore, it also showed strongest inhibitory activities on AChE with IC50 values of 10.0 μM.Download high-res image (220KB)Download full-size image
Co-reporter:Sheng Zhuo Huang, Xing Jie Zhang, Xing Yao Li, Ling Mei Kong, He Zhong Jiang, Qing Yun Ma, Yu Qing Liu, Jiang Miao Hu, Yong Tang Zheng, Yan Li, Jun Zhou, You Xing Zhao
Phytochemistry 2012 Volume 75() pp:99-107
Publication Date(Web):March 2012
DOI:10.1016/j.phytochem.2011.11.013
Seven previously unreported daphnane-type diterpene esters named acutilobins A–G, together with 12 known ones, were isolated from EtOAc extract of Daphne acutiloba Rehd. Their structures were elucidated based on the spectroscopic data. The cytotoxic and anti-HIV-1 activities of these daphnane-type diterpene esters were evaluated through bioassays. Fourteen of these isolates exhibited definite cytotoxic activities against the five human tumor cell lines HL-60, SMMC-7721, A-549, MCF-7, and SW480. Additionally, anti-HIV-1 activities were observed in 13 daphnane-type diterpene esters, among which acutilobins A–G exhibited significant anti-HIV-1 activities with EC50 below 1.5 nM and SI over 10,000. Particularly, genkwanineVIII showed the strongest activity with EC50 0.17 nM and SI 187,010.Graphical abstractSeven daphnane-type diterpene esters acutilobins A–G (1–7) along with 12 known ones (8–19) were isolated from the stems of Daphne acutiloba Rehd. The anti-HIV-1 and cytotoxic bioassay showed that acutilobins A–G exhibited strong anti-HIV-1 activities and definite cytotoxic activities against five human tumor cell lines.Highlights► Seven daphnane-type diterpene ester were identified from Daphne acutiloba. ► Daphnane-type diterpene esters showed strong anti-HIV-1 activity. ► Daphnane-type diterpene esters had the activity to five human tumor cell lines. ►Daphne acutiloba stems could be potential sources of promising anti-HIV-1 drug.
Co-reporter:Peng-Cheng Wang, Xin-Hui Ran, Rui Chen, Liang-Chun Li, Shan-Shan Xiong, Yu-Qing Liu, Huai-Rong Luo, Jun Zhou, You-Xing Zhao
Tetrahedron Letters 2010 Volume 51(Issue 41) pp:5451-5453
Publication Date(Web):13 October 2010
DOI:10.1016/j.tetlet.2010.08.023
Volvalerenone A (1), a new type of mononorsesquiterpenoid with an unprecedented 5/6/6 tricyclic ring system, was isolated from the roots of Valeriana officinalis, the official species of valerian used in Europe. The structure of volvalerenone A was elucidated based on its spectroscopic and single-crystal X-ray crystallography data. The absolute configuration was assigned by the computational method. A possible biosynthetic pathway of volvalerenone A was also proposed. Preliminary biological studies showed that volvalerenone A had weak inhibitory activity on acetylcholine esterase (AChE), and no enhancing activity on nerve growth factor (NGF)-mediated neurite outgrowth in PC12 cells was observed at 50 μM.Volvalerenone A (1), a new type of mononorsesquiterpenoid with an unprecedented 5/6/6 tricyclic ring system, was isolated from the roots of Valeriana officinalis.
Co-reporter:Jun Zhou 周 俊
Chinese Journal of Integrative Medicine 2009 Volume 15( Issue 1) pp:7-12
Publication Date(Web):2009 February
DOI:10.1007/s11655-009-0007-y
Traditional Chinese medicine (TCM) plays an important role in the medical system used in health care and treatment of diseases. This article reviews the basic theory of TCM based on its formation and contributions. Two new personal points are proposed as follows: Six Zang (六脏) theory and deemphasization of the five-element theory. The basic theory of Chinese herbs is also discussed.
Co-reporter:Li-Qin Wang;You-Xing Zhao;JiangMiao Hu;Ai-Qun Jia
Helvetica Chimica Acta 2008 Volume 91( Issue 1) pp:
Publication Date(Web):
DOI:10.1002/hlca.200890007
Abstract
Three new stilbene derivatives, gnetumelin A (1), gnetumelin B (2), and gnetumelin C (3), along with the nine known stilbene derivatives 4–12 were isolated from Gnetum montanumMarkgr. f. megalocarpumMarkgr. Their structures were determined by spectroscopic analysis and comparison of the data with reported ones.
Co-reporter:Li Qin Wang, Jian Hua Wang, Yue Mao Shen, Jun Zhou
Chinese Chemical Letters 2007 Volume 18(Issue 10) pp:1235-1238
Publication Date(Web):October 2007
DOI:10.1016/j.cclet.2007.07.026
Three new C21 steroidal glycosides named inamoside E (1), inamoside F (2) and inamoside G (3) were isolated from the roots of Cynanchum inamoenum (Maxim.) Loes. Their structures were determined by spectroscopic analysis, especially by 1D and 2D NMR experiments.
Co-reporter:Shi-ming DENG, Xian-hui YANG, You-xing ZHAO, Jun ZHOU
Chemical Research in Chinese Universities 2006 Volume 22(Issue 3) pp:400-402
Publication Date(Web):May 2006
DOI:10.1016/S1005-9040(06)60127-X
Co-reporter:Cheng-Sen Li;You-Xing Zhao;Xiao-Dong Luo;Tie-Mei Yi
Helvetica Chimica Acta 2005 Volume 88(Issue 2) pp:325-329
Publication Date(Web):18 FEB 2005
DOI:10.1002/hlca.200590015
Three new urea derivatives, isolated from the Pliocene lignified wood of Pinus armandii, were identified as carbonylbis[imino(6-methyl-3,1-phenylene)]bis[carbamic acid] dimethyl ester (1), and as the corresponding dibutyl ester 2 and bis(2-methylpropyl) ester 3. Their structures were elucidated by spectroscopic methods, including MS and 1D- and 2D-NMR techniques.
Co-reporter:Ai-qun Jia, Ning-hua Tan, Jun Zhou
Fitoterapia (April 2009) Volume 80(Issue 3) pp:192-195
Publication Date(Web):1 April 2009
DOI:10.1016/j.fitote.2009.01.004
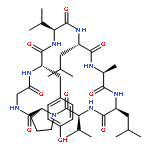
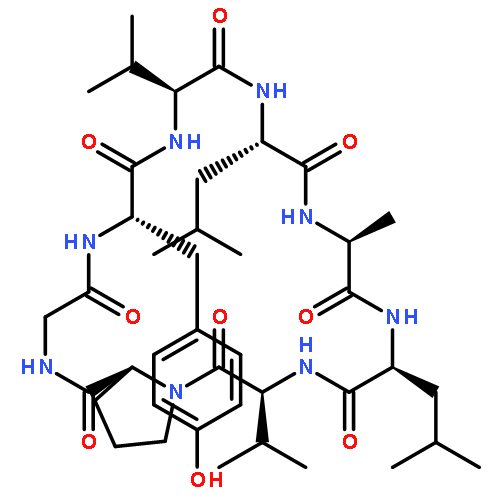
Two new minor cyclopeptides, named japonicin A (1), japonicin B (2), were isolated from the whole plants of Sagina japonica (Caryophyllaceae). Their structures were determined as cyclo-(Pro1-Pro2-Leu2-Leu1-Phe2-Pro3-Gly-Ser-Phe1) (1) and cyclo-(Pro1-Ile-Tyr-Asp-Pro2-Phe2-Pro3-Phe1) (2) on the basis of spectroscopic data, especially by two-dimension NMR technologies.Two new cyclopeptides, sajaponicin A (1), sajaponicin B (2), were isolated from Sagina japonica (Caryophyllaceae). Their structures were determined on the basis of spectroscopic data, especially by two-dimension NMR technologies.Download full-size image



![Phenol, 4-[2-(3,5-dimethoxyphenyl)ethyl]-2-methoxy-](http://img.cochemist.com/ccimg/213900/213842-42-3.png)
![Phenol, 4-[2-(3,5-dimethoxyphenyl)ethyl]-2-methoxy-](http://img.cochemist.com/ccimg/213900/213842-42-3_b.png)
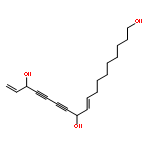
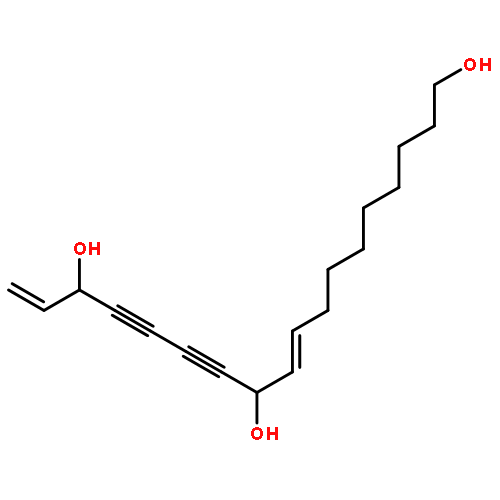
![4-{(1E)-2-[3-(beta-D-glucopyranosyloxy)-5-hydroxyphenyl]ethenyl}-2-methoxyphenyl beta-D-glucopyranoside](http://img.cochemist.com/ccimg/193900/193806-40-5.png)
![4-{(1E)-2-[3-(beta-D-glucopyranosyloxy)-5-hydroxyphenyl]ethenyl}-2-methoxyphenyl beta-D-glucopyranoside](http://img.cochemist.com/ccimg/193900/193806-40-5_b.png)