Co-reporter:Brendan F. Dutter, Laura A. Mike, Paul R. Reid, Katherine M. Chong, Susan J. Ramos-Hunter, Eric P. Skaar, and Gary A. Sulikowski
ACS Chemical Biology 2016 Volume 11(Issue 5) pp:1354
Publication Date(Web):February 18, 2016
DOI:10.1021/acschembio.5b00934
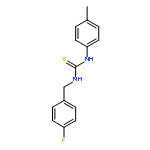
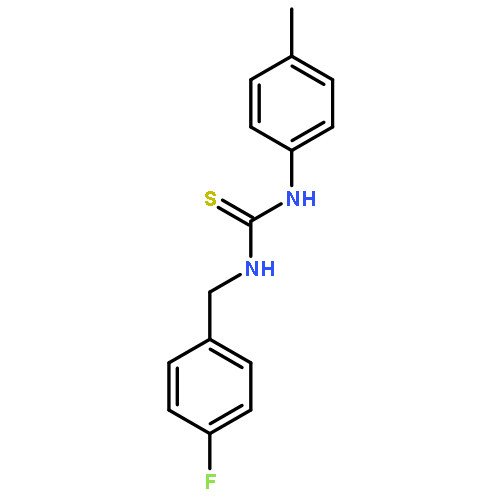
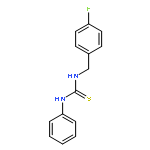
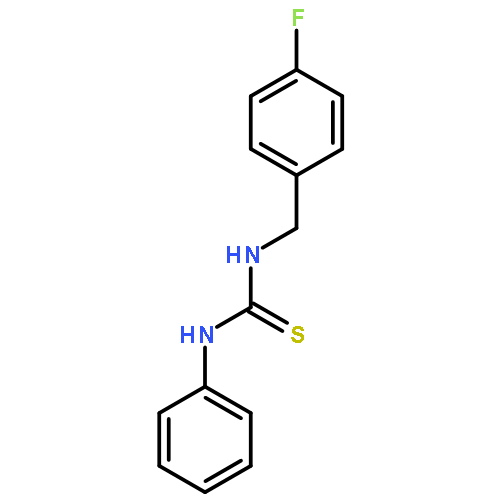
Small molecules active in the pathogenic bacterium Staphylococcus aureus are valuable tools for the study of its basic biology and pathogenesis, and many molecules may provide leads for novel therapeutics. We have previously reported a small molecule, 1, which activates endogenous heme biosynthesis in S. aureus, leading to an accumulation of intracellular heme. In addition to this novel activity, 1 also exhibits toxicity towards S. aureus growing under fermentative conditions. To determine if these activities are linked and establish what features of the molecule are required for activity, we synthesized a library of analogs around the structure of 1 and screened them for activation of heme biosynthesis and anaerobic toxicity to investigate structure–activity relationships. The results of this analysis suggest that these activities are not linked. Furthermore, we have identified the structural features that promote each activity and have established two classes of molecules: activators of heme biosynthesis and inhibitors of anaerobic growth. These molecules will serve as useful probes for their respective activities without concern for the off target effects of the parent compound.
Co-reporter:Katherine M Chong, Nalin Leelatian, Sean M Deguire, Asa A Brockman, David Earl, Rebecca A Ihrie, Jonathan M Irish, Brian O Bachmann and Gary A Sulikowski
The Journal of Antibiotics 2016 69(4) pp:327-330
Publication Date(Web):March 9, 2016
DOI:10.1038/ja.2016.22
The apoptolidins are glycomacrolide microbial metabolites reported to be selectively cytotoxic against tumor cells. Using fluorescently tagged active derivatives we demonstrate selective uptake of these four tagged glycomacrolides in cancer cells over healthy human blood cells. We also demonstrate the utility of these five fluorescently tagged glycomacrolides in fluorescent flow cytometry to monitor cellular uptake of the six glycomacrolides and cellular response.
Co-reporter:Dr. Sean M. DeGuire;David C. Earl;Dr. Yu Du;Brenda A. Crews;Dr. Aaron T. Jacobs;Dr. Alessro Ustione;Cristina Daniel;Katherine M. Chong; Lawrence J. Marnett; David W. Piston; Brian O. Bachmann; Gary A. Sulikowski
Angewandte Chemie International Edition 2015 Volume 54( Issue 3) pp:961-964
Publication Date(Web):
DOI:10.1002/anie.201408906
Abstract
Apoptolidin A has been described among the top 0.1 % most-cell-selective cytotoxic agents to be evaluated in the NCI 60 cell line panel. The molecular structure of apoptolidin A consists of a 20-membered macrolide with mono- and disaccharide moieties. In contrast to apoptolidin A, the aglycone (apoptolidinone) shows no cytotoxicity (>10 μM) when evaluated against several tumor cell lines. Apoptolidin H, the C27 deglycosylated analogue of apoptolidin A, displayed sub-micromolar activity against H292 lung carcinoma cells. Selective esterification of apoptolidins A and H with 5-azidopentanoic acid afforded azido-functionalized derivatives of potency equal to that of the parent macrolide. They also underwent strain-promoted alkyne–azido cycloaddition reactions to provide access to fluorescent and biotin-functionalized probes. Microscopy studies demonstrate apoptolidins A and H localize in the mitochondria of H292 human lung carcinoma cells.
Co-reporter:Dr. Sean M. DeGuire;David C. Earl;Dr. Yu Du;Brenda A. Crews;Dr. Aaron T. Jacobs;Dr. Alessro Ustione;Cristina Daniel;Katherine M. Chong; Lawrence J. Marnett; David W. Piston; Brian O. Bachmann; Gary A. Sulikowski
Angewandte Chemie 2015 Volume 127( Issue 3) pp:975-978
Publication Date(Web):
DOI:10.1002/ange.201408906
Abstract
Apoptolidin A has been described among the top 0.1 % most-cell-selective cytotoxic agents to be evaluated in the NCI 60 cell line panel. The molecular structure of apoptolidin A consists of a 20-membered macrolide with mono- and disaccharide moieties. In contrast to apoptolidin A, the aglycone (apoptolidinone) shows no cytotoxicity (>10 μM) when evaluated against several tumor cell lines. Apoptolidin H, the C27 deglycosylated analogue of apoptolidin A, displayed sub-micromolar activity against H292 lung carcinoma cells. Selective esterification of apoptolidins A and H with 5-azidopentanoic acid afforded azido-functionalized derivatives of potency equal to that of the parent macrolide. They also underwent strain-promoted alkyne–azido cycloaddition reactions to provide access to fluorescent and biotin-functionalized probes. Microscopy studies demonstrate apoptolidins A and H localize in the mitochondria of H292 human lung carcinoma cells.
Co-reporter:Jonathan E. Hempel, Darren W. Engers, Gary A. Sulikowski
Tetrahedron Letters 2014 Volume 55(Issue 13) pp:2157-2159
Publication Date(Web):26 March 2014
DOI:10.1016/j.tetlet.2014.02.069
Efforts toward the synthesis of the decalin ring system common to the hibarimicin shunt metabolite HMP-Y1 and parent aglycone hibarimicinone are reported herein. An intramolecular Diels–Alder cyclization rapidly generated the decalin framework. Two approaches toward completion of the AB decalin were vetted. Incorporation of a phenylsulfonyl leaving group β- to both a ketone and a γ-lactone followed by base-induced elimination of sulfinate led to the undesired α,β-unsaturated lactone. Methanolysis of the γ-lactone followed by elimination produced the unexpected bridged cyclic ether by way of an intramolecular oxy-Michael addition of the endo oriented C13 alcohol.
Co-reporter:Steven D. Townsend and Gary A. Sulikowski
Organic Letters 2013 Volume 15(Issue 19) pp:5096-5098
Publication Date(Web):September 25, 2013
DOI:10.1021/ol402379m
Progress toward the total synthesis of bielschowskysin is described including introduction of the quaternary C12 and neighboring C13 stereocenters.
Co-reporter:Susan J. Ramos-Hunter, Darren W. Engers, Kristian Kaufmann, Yu Du, Craig W. Lindsley, C. David Weaver, Gary A. Sulikowski
Bioorganic & Medicinal Chemistry Letters 2013 Volume 23(Issue 18) pp:5195-5198
Publication Date(Web):15 September 2013
DOI:10.1016/j.bmcl.2013.07.002
This Letter describes a novel series of GIRK activators identified through an HTS campaign. The HTS lead was a potent and efficacious dual GIRK1/2 and GIRK1/4 activator. Further chemical optimization through both iterative parallel synthesis and fragment library efforts identified dual GIRK1/2 and GIRK1/4 activators as well as the first examples of selective GIRK1/4 activators. Importantly, these compounds were inactive on GIRK2 and other non-GIRK1 containing GIRK channels, and SAR proved shallow.
Co-reporter:Aleksandra Baranczak and Gary A. Sulikowski
Organic Letters 2012 Volume 14(Issue 4) pp:1027-1029
Publication Date(Web):February 6, 2012
DOI:10.1021/ol203390w
In the course of studies directed toward the synthesis of dideoxy lomaiviticinone, 3-(nitromethyl)cyclohexenones 2a (X = H) and 2b (X = I) were prepared. The corresponding enolates were reacted with naphthazarin (1) and unexpectedly afforded 1,2-oxazepine 3 and isoxazole 4, respectively. Rationale for their formation is proposed.
Co-reporter:Alexander P. Lamers, Mary E. Keithly, Kwangho Kim, Paul D. Cook, Donald F. Stec, Kelly M. Hines, Gary A. Sulikowski, and Richard N. Armstrong
Organic Letters 2012 Volume 14(Issue 20) pp:5207-5209
Publication Date(Web):October 3, 2012
DOI:10.1021/ol302327t
Bacillithiol (BSH) has been prepared on the gram scale from the inexpensive starting material, d-glucosamine hydrochloride, in 11 steps and 8–9% overall yield. The BSH was used to survey the substrate and metal-ion selectivity of FosB enzymes from four Gram-positive microorganisms associated with the deactivation of the antibiotic fosfomycin. The in vitro results indicate that the preferred thiol substrate and metal ion for the FosB from Staphylococcus aureus are BSH and Ni(II), respectively. However, the metal-ion selectivity is less distinct with FosB from Bacillus subtilis, Bacillus anthracis, or Bacillus cereus.
Co-reporter:Aleksandra Baranczak, Gary A. Sulikowski
Tetrahedron Letters 2012 Volume 53(Issue 11) pp:1345-1346
Publication Date(Web):14 March 2012
DOI:10.1016/j.tetlet.2011.12.126
Co-reporter:Stephen T. Chau, Yoichi Hayakawa, and Gary A. Sulikowski
Organic Letters 2011 Volume 13(Issue 4) pp:756-759
Publication Date(Web):January 19, 2011
DOI:10.1021/ol103003f
The C16−C28 fragment common to the cytotoxic macrolide ammocidin D has been prepared by a stereospecific 5-exo closure of a γ,δ-epoxyketone followed by a rearrangement to a pyran acetal. The reaction pathway was traced by 18O labeling of the keto carbonyl and observation of 18O induced 13C shifts in the pyran acetal product. NMR data of the synthetic C16−C28 fragment compared favorably to the natural product providing support of the assigned stereochemistry.
Co-reporter:Yu Du, Dagmara K. Derewacz, Sean M. Deguire, Jesse Teske, Jacques Ravel, Gary A. Sulikowski, Brian O. Bachmann
Tetrahedron 2011 67(35) pp: 6568-6575
Publication Date(Web):
DOI:10.1016/j.tet.2011.05.106
Co-reporter:Sean M. DeGuire; Shutao Ma; Gary A. Sulikowski
Angewandte Chemie 2011 Volume 123( Issue 42) pp:10114-10116
Publication Date(Web):
DOI:10.1002/ange.201104366
Co-reporter:Sean M. DeGuire; Shutao Ma; Gary A. Sulikowski
Angewandte Chemie International Edition 2011 Volume 50( Issue 42) pp:9940-9942
Publication Date(Web):
DOI:10.1002/anie.201104366
Co-reporter:Brian O. Bachmann, Ruth McNees, Bruce J. Melancon, Victor P. Ghidu, Rachel Clark, Brenda C. Crews, Sean M. DeGuire, Lawrence J. Marnett and Gary A. Sulikowski
Organic Letters 2010 Volume 12(Issue 13) pp:2944-2947
Publication Date(Web):June 1, 2010
DOI:10.1021/ol1009398
The isolation, characterization, and cytotoxicity against H292 cells of apoptolidin G are reported. Apoptolidin G is shown to be derived by a light-induced isomerization of the C2−C3 carbon−carbon double bond of apoptolidin A.
Co-reporter:Brian J. Smith, Tao Qu, Matthew Mulder, Meredith J. Noetzel, Craig W. Lindsley, Gary A. Sulikowski
Tetrahedron 2010 66(26) pp: 4805-4810
Publication Date(Web):
DOI:10.1016/j.tet.2010.03.117
Co-reporter:BrianJ. Smith ;GaryA. Sulikowski
Angewandte Chemie International Edition 2010 Volume 49( Issue 9) pp:1599-1602
Publication Date(Web):
DOI:10.1002/anie.200905732
Co-reporter:BrianJ. Smith ;GaryA. Sulikowski
Angewandte Chemie 2010 Volume 122( Issue 9) pp:1643-1646
Publication Date(Web):
DOI:10.1002/ange.200905732
Co-reporter:Victor P. Ghidu, Ioanna Ntai, Jingqi Wang, Aaron T. Jacobs, Lawrence J. Marnett, Brian O. Bachmann and Gary A. Sulikowski
Organic Letters 2009 Volume 11(Issue 14) pp:3032-3034
Publication Date(Web):June 24, 2009
DOI:10.1021/ol901045v
Glycosylation of a synthetic aglycone using precursor-directed biosynthesis is facilitated by a chemical ketosynthase “knockdown” of the apoptolidin producer Nocardiopsis sp. This synthetic approach facilitated the preparation of an unnatural disaccharide derivative of apoptolidin D that substantially restores cytotoxicity against H292 cells and deconvolutes the role of the decorating sugars in apoptolidin bioactivity.
Co-reporter:Aleksra Baranczak ;GaryA. Sulikowski
Angewandte Chemie International Edition 2009 Volume 48( Issue 33) pp:6005-6007
Publication Date(Web):
DOI:10.1002/anie.200901712
Co-reporter:Aleksra Baranczak ;GaryA. Sulikowski
Angewandte Chemie 2009 Volume 121( Issue 33) pp:6119-6121
Publication Date(Web):
DOI:10.1002/ange.200901712
Co-reporter:Bin Wu;Qingsong Liu
Angewandte Chemie 2004 Volume 116(Issue 48) pp:
Publication Date(Web):9 DEC 2004
DOI:10.1002/ange.200461469
Das komplexe Makrolid Apoptolidinon (1) wurde in 19 Stufen (längste lineare Sequenz) aus (S)-Äpfelsäure hergestellt. Schlüsselreaktionen sind zwei stereoselektive Aldolreaktionen (A), eine Grubbs-Kreuzmetathese zu einem späten Zeitpunkt (B), um ein trisubstituiertes Vinylboronat einzubringen, und eine intramolekulare Suzuki-Miyaura-Reaktion (C).
Co-reporter:Bin Wu;Qingsong Liu
Angewandte Chemie International Edition 2004 Volume 43(Issue 48) pp:
Publication Date(Web):9 DEC 2004
DOI:10.1002/anie.200461469
The complex macrolide apoptolidinone (1) was synthesized in 19 steps (longest linear sequence) from (S)-malic acid. Key reactions include A) two stereoselective aldol reactions, B) a late-stage Grubbs cross-metathesis reaction to install a trisubstituted vinyl boronate, and C) an intramolecular Suzuki–Miyaura reaction.