Co-reporter:Nicholas A. Isley, Ye Wang, Fabrice Gallou, Sachin Handa, Donald H. Aue, and Bruce H. Lipshutz
ACS Catalysis December 1, 2017 Volume 7(Issue 12) pp:8331-8331
Publication Date(Web):November 7, 2017
DOI:10.1021/acscatal.7b03241
Suzuki–Miyaura (SM) cross-couplings of 2-pyridyl MIDA boronates can be successfully carried out in the complete absence of copper by attenuation of the Lewis basicity associated with the pyridyl nitrogen using selected substituents (e.g., fluorine or chlorine) on the ring. This strategy imparts additional synthetic options compared with existing approaches based on the use of Lewis acids or N-oxides. Thus, access to highly valued 2-substituted pyridyl rings via an initial Suzuki–Miyaura coupling can be followed by dehalogenation, SNAr reactions, or a second SM coupling to arrive at 2,6-disubstituted pyridyl arrays, all run in a single pot, enabled by micellar catalysis in water. Accessing targets within drug-like space is demonstrated in a four-step, one-pot sequence. Computational data suggest that the major role being played by electron-withdrawing substituents in promoting these cross-couplings without the need for copper is to slow the rates of protodeboronation of intermediate 2-pyridylboronic acids.Keywords: E factor; green chemistry; micellar catalysis; MIDA boronates; Suzuki−Miyaura;
Co-reporter:Youliang Wang;Akop Yepremyan;Dr. Subir Ghorai;Dr. Robert Todd;Dr. Donald H. Aue;Dr. Liming Zhang
Angewandte Chemie International Edition 2013 Volume 52( Issue 30) pp:7795-7799
Publication Date(Web):
DOI:10.1002/anie.201301057
Co-reporter:Youliang Wang;Akop Yepremyan;Dr. Subir Ghorai;Dr. Robert Todd;Dr. Donald H. Aue;Dr. Liming Zhang
Angewandte Chemie 2013 Volume 125( Issue 30) pp:7949-7953
Publication Date(Web):
DOI:10.1002/ange.201301057
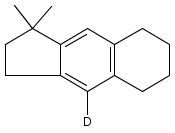
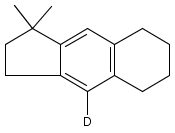
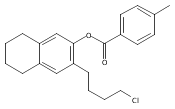
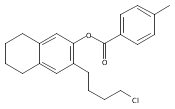
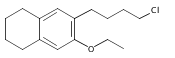
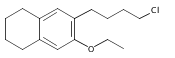
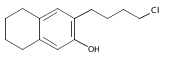
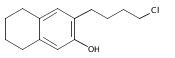
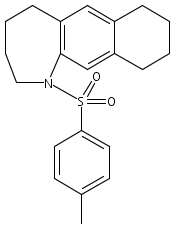
Co-reporter:Karl R. Voigtritter, Nicholas A. Isley, Ralph Moser, Donald H. Aue, Bruce H. Lipshutz
Tetrahedron 2012 68(17) pp: 3410-3416
Publication Date(Web):
DOI:10.1016/j.tet.2011.10.056
Co-reporter:BruceH. Lipshutz ;&x17d;arkoV. Bo&x161;kovi&x107; ;DonaldH. Aue
Angewandte Chemie International Edition 2008 Volume 47( Issue 52) pp:10183-10186
Publication Date(Web):
DOI:10.1002/anie.200804912
Co-reporter:L.D. Betowski, Mark Enlow, Donald H. Aue
International Journal of Mass Spectrometry 2006 Volumes 255–256() pp:123-129
Publication Date(Web):1 September 2006
DOI:10.1016/j.ijms.2006.04.008
Negative-ion chemical-ionization mass spectrometry (NICI MS) has the potential to be a very useful technique in identifying various polycyclic aromatic hydrocarbons (PAHs) in soil and sediment samples. Some PAHs give much stronger signals under NICI MS conditions than others. On the other hand, positive-ion signals are largely comparable under the same source conditions. An extensive set of newly re-evaluated experimental electron affinities (EAs), or free energies of electron attachment, are now available, as well as reliable predicted electron affinities from quantum theoretical calculations or from solution reduction potentials and theoretically predicted solvation energies. In order to show a high negative-ion sensitivity, a PAH must have an EA that exceeds a threshold of approximately of 0.5 eV. Comparisons between the negative-ion to positive-ion sensitivities (N/P ratios) and these new electron affinities show a rough correlation between the two, but naphthacene and perylene are exceptions to this relationship with much lower sensitivities than expected from their high EA values. By calculating the EA for a PAH, one can predict whether a sensitivity enhancement under NICI MS conditions is to be expected. Since aliphatic hydrocarbons and many other substances have negative or very low EAs, NICI MS is expected to be a good technique for detecting PAHs in samples contaminated with other hydrocarbons or compounds with low EAs.
Co-reporter:Donald H. Aue, Michele Guidoni, L.D. Betowski
International Journal of Mass Spectrometry 2000 Volume 201(1–3) pp:283-295
Publication Date(Web):25 July 2000
DOI:10.1016/S1387-3806(00)00210-4
The gas-phase basicities (GBs) of polynuclear aromatic hydrocarbons (PAHs) have been calculated using the semiempirical Austin model 1 method, ab initio quantum mechanical methods at the HF/3-21G, HF/6-31G(d), MP2/6-31G(d), MP2/6-31+G(d,p) levels, and the B3LYP/6-311G(d,p) density functional method. GBs calculated at these levels of theory are compared to experimentally known GBs measured by equilibrium mass spectrometric methods. Theoretically calculated entropies for PAHs and their protonated carbocations are used to reevaluate original experimental equilibrium data producing a revised set of experimental GBs and proton affinities for PAHs and related hydrocarbons. Experimental GBs for 40 hydrocarbons are found to correlate well with AM1, Hartree–Fock, DFT and MP2 calculated GBs, with regression analyses showing standard errors of 2.12, 1.53, 1.36, and 1.55 kcal mol−1 for each of the four methods, respectively. The results permit an evaluation of the reliability of experimental data and suggest a need for new experimental work for some molecules. Predictions are made for GBs for 12 new PAHs whose GBs have not yet been measured.
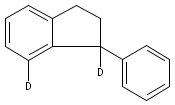
Co-reporter:Longwu Ye ; Yanzhao Wang ; Donald H. Aue ;Liming Zhang
Journal of the American Chemical Society () pp:
Publication Date(Web):December 14, 2011
DOI:10.1021/ja2091992
Facile cycloisomerization of (2-ethynylphenyl)alkynes is proposed to be promoted synergistically by two molecules of BrettPhosAuNTf2, affording tricyclic indenes in mostly good yields. A gold vinylidene is most likely generated as one of the reaction intermediates on the basis of both mechanistic studies and theoretical calculations. Different from the well-known Rh, Ru, and W counterparts, this novel gold species is highly reactive and undergoes facile intramolecular C(sp3)–H insertions as well as O–H and N–H insertions. The formation step for the gold vinylidene is predicted theoretically to be complex with a bifurcated reaction pathway. A pyridine N-oxide acts as a weak base to facilitate the formation of an alkynylgold intermediate, and the bulky BrettPhos ligand in the gold catalyst likely plays a role in sterically steering the reaction toward formation of the gold vinylidene.