Co-reporter:Mo Wang;Xiao Zhang;Zheng Ling;Wanbin Zhang
Chemical Communications 2017 vol. 53(Issue 8) pp:1381-1384
Publication Date(Web):2017/01/24
DOI:10.1039/C6CC09451A
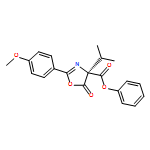
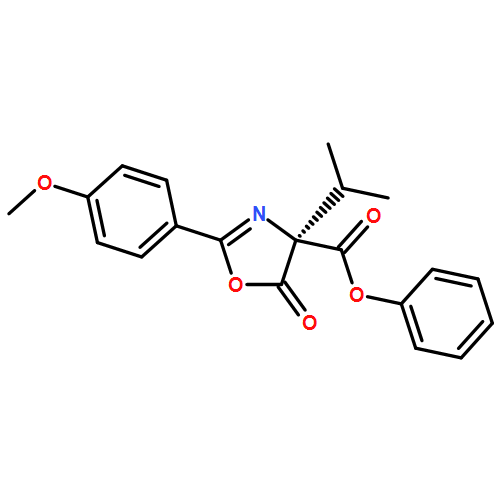
A direct enantioselective C-acylation of 3-substituted benzofuran-2(3H)-ones (with up to 97% ee) was developed using a chiral bicyclic imidazole catalyst OAc-DPI and an achiral tertiary amine additive DIPEA. The reaction mechanism for the direct C-acylation has been investigated using both experimental and theoretical studies.
Co-reporter:Yang Liu;Boqiang Ding;Delong Liu
Research on Chemical Intermediates 2017 Volume 43( Issue 8) pp:4959-4966
Publication Date(Web):07 April 2017
DOI:10.1007/s11164-017-2923-6
Chiral organophosphorus compounds possessing a P-stereogenic center have been widely used as agricultural chemicals, pharmaceuticals, organocatalysts, and ligands for transition-metal catalysis. P-Stereogenic intermediates bearing a tert-butyl(methyl)phosphino group are important for the preparation of several commonly used diphosphine ligands. However, previously reported synthetic methods used hazardous starting materials and are difficult to operate. In order to overcome these limitations, a new and convenient synthesis for a number of P-stereogenic intermediates possessing a tert-butyl(methyl)phosphino group has been developed. The reported route relies on an air- and moisture-stable secondary phosphine oxide prepared from a readily available starting material, trichlorophosphane.
Co-reporter:Qiupeng Hu, Jianzhong Chen, Zhenfeng Zhang, Yangang Liu, and Wanbin Zhang
Organic Letters 2016 Volume 18(Issue 6) pp:1290-1293
Publication Date(Web):March 8, 2016
DOI:10.1021/acs.orglett.6b00212
Catalyzed by a rhodium complex of P-stereogenic diphosphine ligand (R)-2-tert-butylmethylphosphino-3-(di-tert-butylphosphino)quinoxaline ((R)-3H-QuinoxP*), five-membered cyclic α-dehydroamino ketones bearing endocyclic vinyl and endocyclic keto-carbonyl groups were sequentially hydrogenated to give chiral cyclic trans-β-amino alcohols with two contiguous stereocenters in quantitative conversions, excellent enantioselectivities and good diastereoselectivities.
Co-reporter:Yuping Hong, Jianzhong Chen, Zhenfeng Zhang, Yangang Liu, and Wanbin Zhang
Organic Letters 2016 Volume 18(Issue 11) pp:2640-2643
Publication Date(Web):May 23, 2016
DOI:10.1021/acs.orglett.6b01073
Via a strategy of asymmetric reductive desymmetrization, chiral cis-epoxy naphthoquinols with multiple contiguous stereocenters and functional groups were synthesized with excellent enantioselectivities (96–99% ee) and diastereoselectivities (8/1–15/1). A combined asymmetric hydrogenation/transfer hydrogenation mechanism was proposed based on experimental results.
Co-reporter:Dongyang Fan, Jian Lu, Yang Liu, Zhenfeng Zhang, Yangang Liu, Wanbin Zhang
Tetrahedron 2016 Volume 72(Issue 35) pp:5541-5547
Publication Date(Web):1 September 2016
DOI:10.1016/j.tet.2016.07.046
Catalyzed by a rhodium complex of P-stereogenic diphosphine ligand trichickenfootphos (TCFP), asymmetric hydrogenation of racemic aldimines via dynamic kinetic resolution has been realized for the preparation of chiral arylglycines with good yields and enantioselectivities.
Co-reporter:Mo Wang, Xiao Zhang, Zheng Ling, Zhenfeng Zhang and Wanbin Zhang
Chemical Communications 2017 - vol. 53(Issue 8) pp:NaN1384-1384
Publication Date(Web):2017/01/03
DOI:10.1039/C6CC09451A
A direct enantioselective C-acylation of 3-substituted benzofuran-2(3H)-ones (with up to 97% ee) was developed using a chiral bicyclic imidazole catalyst OAc-DPI and an achiral tertiary amine additive DIPEA. The reaction mechanism for the direct C-acylation has been investigated using both experimental and theoretical studies.

![(7R)-6,7-dihydro-7-methoxy-5H-Pyrrolo[1,2-a]imidazole](http://img.cochemist.com/ccimg/1221200/1221187-75-2.png)
![(7R)-6,7-dihydro-7-methoxy-5H-Pyrrolo[1,2-a]imidazole](http://img.cochemist.com/ccimg/1221200/1221187-75-2_b.png)
![(7R)-7-ethoxy-6,7-dihydro-5H-Pyrrolo[1,2-a]imidazole](http://img.cochemist.com/ccimg/1221200/1221187-76-3.png)
![(7R)-7-ethoxy-6,7-dihydro-5H-Pyrrolo[1,2-a]imidazole](http://img.cochemist.com/ccimg/1221200/1221187-76-3_b.png)











![Oxazole, 2-[2'-[bis[4-(trifluoromethyl)phenyl]phosphino][1,1'-biphenyl]-2-yl]-4,5-dihydro-4-phenyl-, (4S)-](/data/chemimg/3721700/1266398-39-3.png)
![Oxazole, 2-[2'-[bis[4-(trifluoromethyl)phenyl]phosphino][1,1'-biphenyl]-2-yl]-4,5-dihydro-4-phenyl-, (4S)-](/data/chemimg/3721700/1266398-39-3_b.png)