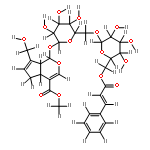
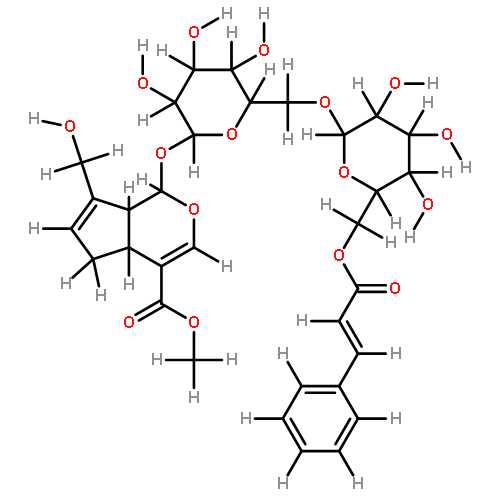
An HPLC with SPE method has been developed for analysis of constituents in rat blood after oral administration of the extract of Acanthopanax senticosus (ASE). The plasma sample was prepared by SPE method equipped with Oasis HLB cartridge (3cc, 60 mg). The analysis was performed on a Dikma Diamonsil RP18 column (4.6 mm×150 mm, 5 μm) with the gradient elution of solvent A (ACN) and solvent B (0.1% aqueous phosphoric acid, v/v) and the detection wavelength was set at 270 nm. The calibration curve was linear over the range of 0.156–15.625 μg/mL. The LOD was 60 ng/mL. The intraday precision was less than 5.80%, and the interday precision was less than 6.0%. The recovery was (87.30 ± 1.73)%. As a result, 19 constituents were detected in rat plasma after oral administration of the ASE, including 11 original compounds in ASE and eight metabolites, and three of the metabolites originated from syringin in ASE. Six constituents were identified by comparing with the corresponding reference compounds.

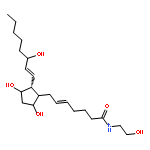
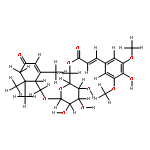
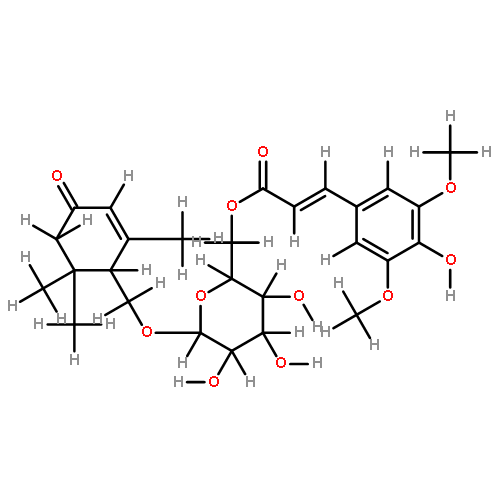
![(4S)-3-{{{6-O-[(2E)-3-(4-hydroxy-3,5-dimethoxyphenyl)-1-oxoprop-2-en-1-yl]-beta-D-glucopyranosyl}oxy}methyl}-4-(hydroxymethyl)-5,5-dimethylcyclohex-2-en-1-one](http://img.cochemist.com/ccimg/1229700/1229609-47-5.png)
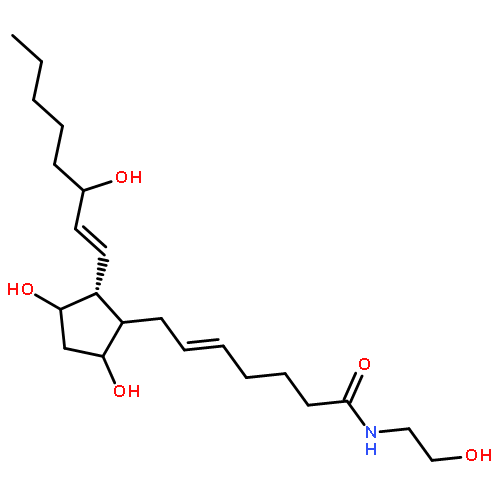
![(4S)-3-{{{6-O-[(2E)-3-(4-hydroxy-3,5-dimethoxyphenyl)-1-oxoprop-2-en-1-yl]-beta-D-glucopyranosyl}oxy}methyl}-4-(hydroxymethyl)-5,5-dimethylcyclohex-2-en-1-one](http://img.cochemist.com/ccimg/1229700/1229609-47-5_b.png)
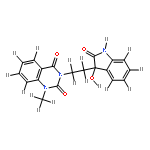
![INDOLO[2',3':3,4]PYRIDO[2,1-B]QUINAZOLIN-5(14H)-ONE, 14-METHYL-](http://img.cochemist.com/ccimg/878700/878628-80-9.png)
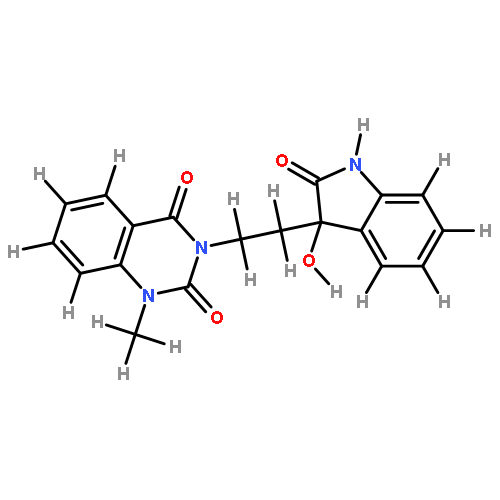
![INDOLO[2',3':3,4]PYRIDO[2,1-B]QUINAZOLIN-5(14H)-ONE, 14-METHYL-](http://img.cochemist.com/ccimg/878700/878628-80-9_b.png)