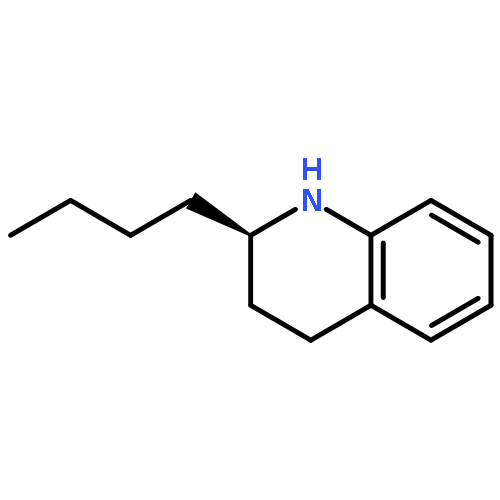
Co-reporter:Yin-Suo Lu and Wing-Yiu Yu
Organic Letters 2016 Volume 18(Issue 6) pp:1350-1353
Publication Date(Web):February 29, 2016
DOI:10.1021/acs.orglett.6b00283
A Cp*Rh(III)-catalyzed cross-coupling of alkyltrifluoroborate with α-diazomalonates was developed; the C(sp3)–C(sp3) bond coupled products were formed in up to 97% yields. The reaction tolerates some useful functional groups, including ketone, ester, amide, ether, sulfonyl, and thiophene. Electrospray ionization mass spectrometry (ESI-MS) analysis revealed the formation of a distinct molecular species corresponding to σ-alkylrhodium(III) complexes. The successful diazo coupling reaction may be attributed to coordination of the amide group that promotes stability of the alkylrhodium(III) complex through the formation of a five-membered metallacycle.
Co-reporter:Yan-Fung Lau, Chun-Ming Chan, Zhongyuan Zhou and Wing-Yiu Yu
Organic & Biomolecular Chemistry 2016 vol. 14(Issue 28) pp:6821-6825
Publication Date(Web):16 Jun 2016
DOI:10.1039/C6OB00719H
A [Cp*Rh(III)]-catalyzed electrophilic amination of arylboronic acids with diethyl azodicarboxylate (DEAD) was developed, and arylhydrazides were produced in excellent yields and selectivity. The analogous amination with the arylazocarboxylates afforded the corresponding N,N-diarylhydrazides. The electrophilic amination of arylboronic acids with azocarboxylates proceeds readily under mild conditions with excellent functional group tolerance. Up to 99% yields were obtained. Preliminary mechanistic studies revealed that prior formation of an arylrhodium(III) intermediate for the azo coupling reaction can be ruled out.
Co-reporter:Fo-Ning Ng, Yan-Fung Lau, Zhongyuan Zhou, and Wing-Yiu Yu
Organic Letters 2015 Volume 17(Issue 7) pp:1676-1679
Publication Date(Web):March 16, 2015
DOI:10.1021/acs.orglett.5b00440
A Rh(III)-catalyzed cascade arylation and chlorination of α-diazocarbonyl compounds with arylboronic acids and N-chlorosuccinimide was achieved. The reaction exhibits excellent functional group tolerance on the organoboron and the diazo reagents; the functionalized α-aryl-α-chlorocarbonyl compounds were obtained in up to 86% yields. The cascade reaction should involve migratory carbene insertion of arylrhodium(III) to form some reactive rhodium(III)–diketonate complexes. Its subsequent reaction with N-chlorosuccinmide afforded the α-chlorocarbonyl products.
Co-reporter:Chun-Wo Chan, Pui-Yiu Lee, Wing-Yiu Yu
Tetrahedron Letters 2015 Volume 56(Issue 20) pp:2559-2563
Publication Date(Web):13 May 2015
DOI:10.1016/j.tetlet.2015.03.109
A Cu-catalyzed cross-dehydrogenative coupling of N-arylacrylamides with chloroform is achieved using tert-butyl peroxybenzoate as oxidant, and trichloromethylated oxindoles were obtained in excellent yield and regioselectivity. This reaction should proceed by cascade CCl3 radical CC addition and aryl cyclization.
Co-reporter:Hon-Wah Lam, Ka-Yi Man, Wai-Wing Chan, Zhongyuan Zhou and Wing-Yiu Yu
Organic & Biomolecular Chemistry 2014 vol. 12(Issue 24) pp:4112-4116
Publication Date(Web):01 Apr 2014
DOI:10.1039/C4OB00512K
A Rh(III)-catalyzed oxidative [4 + 1] cycloaddition of benzohydroxamic acids and α-diazoesters is achieved to afford benzolactams in up to 93% yields. With the N-OAc amido moiety as a directing group, the ortho-C–H is selectively functionalized and the catalytic reaction exhibits excellent tolerance to different functional substituents. A notable rhodacyclic complex is isolated and structurally characterized, suggesting that C–H/N–H cyclometallation is a key step in the catalytic cycle.
Co-reporter:Fo-Ning Ng;Dr. Zhongyuan Zhou ;Dr. Wing-Yiu Yu
Chemistry - A European Journal 2014 Volume 20( Issue 15) pp:4474-4480
Publication Date(Web):
DOI:10.1002/chem.201304855
Abstract
A RhIII-catalyzed direct ortho-CH amidation/amination of benzoic acids with N-chlorocarbamates/N-chloromorpholines was achieved, giving anthranilic acids in up to 85 % yields with excellent ortho-selectivity and functional-group tolerance. Successful benzoic acid aminations were achieved with carbamates bearing various amide groups including NHCO2Me, NHCbz, and NHTroc (Cbz=carbobenzyloxy; Troc=trichloroethylchloroformate), as well as secondary amines, such as morpholines, piperizines, and piperidines, furnishing highly functionalized anthranilic acids. A stoichiometric reaction of a cyclometallated rhodium(III) complex of benzo[h]quinoline with a silver salt of N-chlorocarbamate afforded an amido–rhodium(III) complex, which was isolated and structurally characterized by X-ray crystallography. This finding confirmed that the CN bond formation results from the cross-coupling of N-chlorocarbamate with the aryl–rhodium(III) complex. Yet, the mechanistic details regarding the CN bond formation remain unclear; pathways involving 1,2-aryl migration and rhodium(V)– nitrene are plausible.
Co-reporter:Wai-Wing Chan, Zhongyuan Zhou and Wing-Yiu Yu
Chemical Communications 2013 vol. 49(Issue 74) pp:8214-8216
Publication Date(Web):16 Jul 2013
DOI:10.1039/C3CC44769C
A Pd(II)-catalyzed oxidative direct C(sp2)–H/C(sp3)–H cross coupling of anilides with α-dicarbonyl compounds with Mn(OAc)3·2H2O as the oxidant is reported and this protocol provides facile access to α-aryl malonates and β-keto esters in good yields and regioselectivity.
Co-reporter:Ka-Ho Ng, Zhongyuan Zhou and Wing-Yiu Yu
Chemical Communications 2013 vol. 49(Issue 63) pp:7031-7033
Publication Date(Web):29 May 2013
DOI:10.1039/C3CC42937G
Rh(III)-catalyzed aromatic C–H amination of acetophenone o-methyloximes with primary N-chloroalkylamines was developed, and the arylamine products were obtained in up to 92% yield. The reaction probably involves rate-limiting electrophilic C–H bond cleavage (kH/kD = 2).
Co-reporter:Wai-Wing Chan ; Siu-Fung Lo ; Zhongyuan Zhou
Journal of the American Chemical Society 2012 Volume 134(Issue 33) pp:13565-13568
Publication Date(Web):August 3, 2012
DOI:10.1021/ja305771y
A Rh-catalyzed intermolecular coupling of diazomalonates with arene C–H bonds is reported. The reaction is initiated by electrophilic C–H activation, which is followed by coupling of the arylrhodium(III) complex with the diazomalonate. In most cases, arenes with oximes, carboxylic acids, and amines as directing groups cross-couple with diazomalonates with excellent regioselectivities and functional group tolerance, and thus, this reaction offers a new route to α-aryl carbonyl compounds for specific applications.
Co-reporter:Ka-Ho Ng, Fo-Ning Ng and Wing-Yiu Yu
Chemical Communications 2012 vol. 48(Issue 95) pp:11680-11682
Publication Date(Web):16 Oct 2012
DOI:10.1039/C2CC36502B
An efficient method for synthesis of anthranilic acids by Pd-catalyzed ortho-C–H amidation of benzoic acids is disclosed. The amidation is proposed to proceed by carboxylate-assisted ortho-C–H palladation to form an arylpalladium(II) complex, followed by nitrene insertion to the Pd–C bond.
Co-reporter:Yan-Fung Lau, Chun-Ming Chan, Zhongyuan Zhou and Wing-Yiu Yu
Organic & Biomolecular Chemistry 2016 - vol. 14(Issue 28) pp:NaN6825-6825
Publication Date(Web):2016/06/16
DOI:10.1039/C6OB00719H
A [Cp*Rh(III)]-catalyzed electrophilic amination of arylboronic acids with diethyl azodicarboxylate (DEAD) was developed, and arylhydrazides were produced in excellent yields and selectivity. The analogous amination with the arylazocarboxylates afforded the corresponding N,N-diarylhydrazides. The electrophilic amination of arylboronic acids with azocarboxylates proceeds readily under mild conditions with excellent functional group tolerance. Up to 99% yields were obtained. Preliminary mechanistic studies revealed that prior formation of an arylrhodium(III) intermediate for the azo coupling reaction can be ruled out.
Co-reporter:Hon-Wah Lam, Ka-Yi Man, Wai-Wing Chan, Zhongyuan Zhou and Wing-Yiu Yu
Organic & Biomolecular Chemistry 2014 - vol. 12(Issue 24) pp:NaN4116-4116
Publication Date(Web):2014/04/01
DOI:10.1039/C4OB00512K
A Rh(III)-catalyzed oxidative [4 + 1] cycloaddition of benzohydroxamic acids and α-diazoesters is achieved to afford benzolactams in up to 93% yields. With the N-OAc amido moiety as a directing group, the ortho-C–H is selectively functionalized and the catalytic reaction exhibits excellent tolerance to different functional substituents. A notable rhodacyclic complex is isolated and structurally characterized, suggesting that C–H/N–H cyclometallation is a key step in the catalytic cycle.
Co-reporter:Ka-Ho Ng, Fo-Ning Ng and Wing-Yiu Yu
Chemical Communications 2012 - vol. 48(Issue 95) pp:NaN11682-11682
Publication Date(Web):2012/10/16
DOI:10.1039/C2CC36502B
An efficient method for synthesis of anthranilic acids by Pd-catalyzed ortho-C–H amidation of benzoic acids is disclosed. The amidation is proposed to proceed by carboxylate-assisted ortho-C–H palladation to form an arylpalladium(II) complex, followed by nitrene insertion to the Pd–C bond.
Co-reporter:Wai-Wing Chan, Zhongyuan Zhou and Wing-Yiu Yu
Chemical Communications 2013 - vol. 49(Issue 74) pp:NaN8216-8216
Publication Date(Web):2013/07/16
DOI:10.1039/C3CC44769C
A Pd(II)-catalyzed oxidative direct C(sp2)–H/C(sp3)–H cross coupling of anilides with α-dicarbonyl compounds with Mn(OAc)3·2H2O as the oxidant is reported and this protocol provides facile access to α-aryl malonates and β-keto esters in good yields and regioselectivity.
Co-reporter:Ka-Ho Ng, Zhongyuan Zhou and Wing-Yiu Yu
Chemical Communications 2013 - vol. 49(Issue 63) pp:NaN7033-7033
Publication Date(Web):2013/05/29
DOI:10.1039/C3CC42937G
Rh(III)-catalyzed aromatic C–H amination of acetophenone o-methyloximes with primary N-chloroalkylamines was developed, and the arylamine products were obtained in up to 92% yield. The reaction probably involves rate-limiting electrophilic C–H bond cleavage (kH/kD = 2).