Co-reporter:Yongbin Tong, Weitong Xu, Yan Wu, Liting Ou, Mian Zhang, Xianghong Xu, Chaofeng Zhang
Journal of Pharmaceutical and Biomedical Analysis 2017 Volume 141(Volume 141) pp:
Publication Date(Web):15 July 2017
DOI:10.1016/j.jpba.2017.04.018
•Neotuberostemonine (NS) and tuberostemonine (TS) is a pair of stereoisomers.•NS produced 48 phase I (hydrolyzed or/and hydroxylated) metabolites in rats.•TS yielded 23 phase I and 9 phase II (GluA and GSH conjugated) metabolites in rats.•The structures of all metabolites were elucidated in detail.Neotuberostemonine (NS) and tuberostemonine (TS), a pair of stereoisomers, are the active components contained in Stemona tuberosa, an antitussive herbal medicine in China. Two isomers have different pharmacological efficacies, which will be related with their in vivo disposition. However, the metabolic fates of NS and TS remain unknown. A method of high performance liquid chromatography/quadrupole time-of-flight mass spectrometry coupled with mass detect filter technique was established to investigate the metabolites in rat plasma, bile, urine, and feces after oral administration of the equal doses of NS and TS. The results showed that NS produced 48 phase I metabolites, including NS, 3 hydrolyzed, 14 hydroxylated, 20 monohydrolyzed + hydroxylated and 10 dihydrolyzed + hydroxylated metabolites. The number of detected NS metabolites was 11, 39, 22 and 30 in plasma, bile, urine and feces. TS yielded 23 phase I metabolites, including TS, 3 hydrolyzed, 7 hydroxylated, 9 monohydrolyzed + hydroxylated and 3 dihydrolyzed + hydroxylated metabolites. Besides, TS yielded 9 phase II metabolites, including 1 glucuronic acid and 2 glutathione conjugates, and the later further degraded and modified into cysteine-glycine, cysteine and N-acetylcysteine conjugates. The number of detected TS metabolites was 9, 24, 24 and 15 in plasma, bile, urine and feces. Different metabolic patterns may be one of the main reasons leading to different pharmacological effects of NS and TS.Download high-res image (265KB)Download full-size image
Co-reporter:Dong-Xia Zhao;Bing-Qiang Hu;Chao-Feng Zhang;Xiang-Hong Xu
Journal of Separation Science 2015 Volume 38( Issue 4) pp:571-575
Publication Date(Web):
DOI:10.1002/jssc.201401008
We established a qualitative method to analyze the main chemical compositions of the root of Aster tataricus. Most of the peaks were separated on a C18 column packed with 5.0 μm particles, and 28 compounds were identified, including 11 chlorogenic acids, ten astins/asterinins, and seven astersaponins, four of which were reported for the first time from A. tataricus. Furthermore, we developed a reliable method for the simultaneous quantification of 3-caffeoylquinic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, astin A, astin B, astin C, astersaponin A, and astersaponin C, and the qualified separations were achieved only on a C18 column packed with 2.7 μm particles. The method was used to measure the concentrations of eight components in samples from two major producing areas in China, and the average contents in samples from Bozhou (Anhui) were higher than those in samples from Anguo (Hebei).
Co-reporter:Yu Gao, Jun Wang, Chao-Feng Zhang, Xiang-Hong Xu, Mian Zhang, Ling-Yi Kong
Tetrahedron 2014 70(4) pp: 967-974
Publication Date(Web):
DOI:10.1016/j.tet.2013.12.003
Co-reporter:Yan Wu, Liting Ou, Dong Han, Yongbin Tong, Mian Zhang, Xianghong Xu, Chaofeng Zhang
Fitoterapia (July 2016) Volume 112() pp:22-29
Publication Date(Web):1 July 2016
DOI:10.1016/j.fitote.2016.05.003
Neotuberostemonine is a potent antitussive alkaloid extracted from Stemona tuberosa. However, the pharmacokinetics, tissue distribution and excretion of pure neotuberostemonine have not been reported. The present study was aimed to investigate the pharmacokinetic parameters of neotuberostemonine by developing an ultra-high performance liquid chromatography–tandem mass spectrometry method. Neotuberostemonine and tetrahydropalmatine (internal standard, IS) in bio-samples were extracted by protein precipitation with methanol and successfully separated on a Zorbax Extend C18 column by using a mobile phase of acetonitrile and a mixture of 0.1% formic acid and 5 mM ammonium acetate. The detection was performed by using positive ion electrospray ionization in multiple reaction monitoring mode. The MS/MS ion transitions were monitored at m/z 376.1 → 302.0 for neotuberostemonine and 355.8 → 192.0 for IS. After oral administration of neotuberostemonine in rats, the Cmax and AUC0–∞ were 11.37 ng/mL and 17.68 ng·h/mL at 20 mg/kg and 137.6 ng/mL and 167.4 ng·h/mL at 40 mg/kg, and the t1/2 were 2.28 and 3.04 h at 20 and 40 mg/kg, respectively. The high neotuberostemonine concentrations were found in intestine, stomach and liver, and there was no long-term accumulation of neotuberostemonine in tissues. Total recoveries of neotuberostemonine were only 0.90% (0.19% in bile, 0.05% in urine and 0.66% in feces), which might be resulted from the intestine and liver first-pass effects, indicating that neotuberostemonine may be mainly excreted as its metabolites. All above results would provide helpful information for the further pharmacological and clinical studies of neotuberostemonine and the crude drug.Download high-res image (348KB)Download full-size image
Co-reporter:Li Wang, Ming-Dan Li, Pei-Pei Cao, Chao-Feng Zhang, Fang Huang, Xiang-Hong Xu, Bao-Lin Liu, Mian Zhang
Chemico-Biological Interactions (5 November 2014) Volume 223() pp:1-9
Publication Date(Web):5 November 2014
DOI:10.1016/j.cbi.2014.09.003
•Astin B, a halogenated cyclopentapeptide, is hepatotoxic both in vitro & in vivo.•Astin B can provoke oxidative stress and mediate apoptosis in vitro.•Astin B can also induce autophagy in vitro to protect cells from apoptosis.•Astin B is one of the main hepatotoxic substances contained in Aster tataricus.Astins (including astin B) are a class of halogenated cyclic pentapeptides isolated from the medicinal herb of Aster tataricus. However, our previous works showed that the herbal medicine was hepatotoxic in vivo, and a toxicity-guided isolation method led to the identification of a cyclopeptide astin B. Astin B is structurally similar to cyclochlorotine, a well-known hepatotoxic mycotoxin. Thus, the aim of this study was to determine the potential cytotoxic effects and the underlying mechanism of astin B on human normal liver L-02 cells. We found that astin B has hepatotoxic effects in vitro and in vivo and that hepatic injury was primarily mediated by apoptosis in a mitochondria/caspase-dependent manner. Astin B provoked oxidative stress-associated inflammation in hepatocytes as evidenced by increased levels of reactive oxygen species (ROS), reduced contents of intracellular glutathione (GSH), and enhanced phosphorylation of c-Jun N-terminal kinase (JNK). Furthermore, the mitochondria-dependent apoptosis was evidenced by the depolarization of the mitochondrial membrane potential, the release of cytochrome c into cytosol, the increased ratio of Bax/Bcl-2, and the increased activities of caspases-9 and -3. Interestingly, astin B treatment also induces autophagy in L-02 cells, characterized by acidic-vesicle fluorescence, increased LC3-II and decreased p62 expression. Autophagy is a protective mechanism that is used to protect cells from apoptosis. The presence of autophagy is further supported by the increased cytotoxicity and the enhanced cleaved caspase-3 after co-treatment of cells with an autophagy inhibitor, also by increased LC3-II and decreased p62 after co-treatment with a caspase inhibitor. Taken together, astin B, most likely together with other members of astins, is the substance that is primarily responsible for the hepatotoxicity of A. tataricus.Download full-size image
Co-reporter:Fan Xie, Mian Zhang, Chao-Feng Zhang, Zheng-Tao Wang, Bo-Yang Yu, Jun-Ping Kou
Journal of Ethnopharmacology (22 May 2008) Volume 117(Issue 3) pp:463-466
Publication Date(Web):22 May 2008
DOI:10.1016/j.jep.2008.02.025
Aim of studyThe fruit of Melia toosendan Sieb. et Zucc. (MTF) is a traditional Chinese herbal medicine in the treatment of stomachache and many acute or chronic inflammations, as well as ascariasis. This paper aimed to investigate the anti-inflammatory and analgesic activities of the MTF extract and two main limonoid-type triterpenoids isolated from MTF.Materials and methodsThe ethanolic extract of MTF and two limonoids, isotoosendanin (1) and 1-O-tigloyl-1-O-debenzoylohchinal (2) were evaluated for their anti-inflammatory and analgesic activities. Acetic acid-induced vascular permeability and λ-carrageenan-induced hind paw edema tests in mice were used to investigate anti-inflammatory activity; and acetic acid-induced writhing and hot-plate tests in mice were used to determine analgesic effect.ResultsBoth the ethanolic extract and two limonoids displayed significant anti-inflammatory effects. Although the ethanolic extract showed remarkable analgesic effects in both writhing and hot-plate tests, the two limonoids had analgesic effects just in writhing test.ConclusionThe results suggested that the ethanolic extract of MTF had obvious anti-inflammatory and analgesic activities, and the two limonoids were the active constituents contributing to the anti-inflammatory and analgesic effects of MTF.


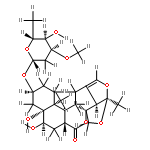
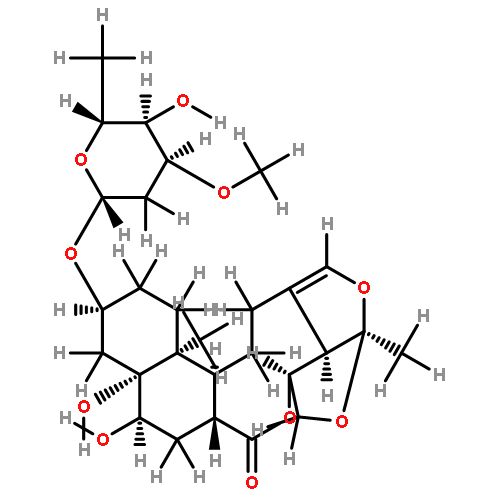


![(3S,3aR,3bS,5S,6R,10aR,11R,11aR)-11-ethyldecahydro-3b-hydroxy-3-methyl-5-[(2S,4S)-tetrahydro-4-methyl-5-oxofuran-2-yl]-6,10a-methanofuro[2',3':4,5]cyclopent[1,2-d]azonine-2,12(3H)-dione](http://img.cochemist.com/ccimg/1219800/1219704-25-2.png)
![(3S,3aR,3bS,5S,6R,10aR,11R,11aR)-11-ethyldecahydro-3b-hydroxy-3-methyl-5-[(2S,4S)-tetrahydro-4-methyl-5-oxofuran-2-yl]-6,10a-methanofuro[2',3':4,5]cyclopent[1,2-d]azonine-2,12(3H)-dione](http://img.cochemist.com/ccimg/1219800/1219704-25-2_b.png)