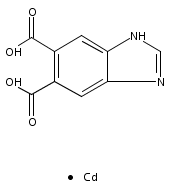
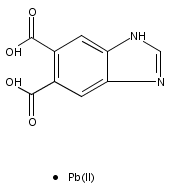
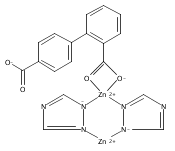
Series of mixed valence monophosphates AFe3-xMgx(PO4)3 [A = Sr(x = 0), Ba(x = 0.6), Pb(x = 0.6)] were synthesized by mild hydrothermal treatment at 210 °C. Refinements of single crystal X-ray diffraction datas show all these compounds are isostructural. The attempts to make AFe3(PO4)3 (A = Ba, Pb) hydrothermally in the experiment were unsuccessful. However, the Mg-doped homologues AFe2.4Mg0.6(PO4)3 (A = Ba, Pb) were synthesized with the addition of MgCO3 in the reactants as mineralizer. EDS and single crystal X-ray data refinement indicated that the Mg2+ cations were doped in the Fe2+ sites of AFe2.4Mg0.6(PO4)3 (A = Ba, Pb). The influence of the Mg-doping on the structure and the reason why the Mg doped in the Fe(II) site instead of A site was discussed from the point of view of the bond valence model.