Co-reporter:Wenhua Huang, Hong-Ying Rong, and Jie Xu
The Journal of Organic Chemistry 2015 Volume 80(Issue 13) pp:6628-6638
Publication Date(Web):June 12, 2015
DOI:10.1021/acs.joc.5b01031
Cyclic α-alkoxyphosphonium salts have been synthesized from (2-(diphenylphosphino)phenyl)methanol and aldehydes in 36–89% yields. These phosphonium salts are bench-stable solids and undergo Wittig olefination with aldehydes under basic conditions (K2CO3 or t-BuOK) to form benzylic vinyl ethers, which are readily hydrolyzed to 1,2-disubstituted ethanones under acidic conditions. The formation mechanism of these phosphonium salts via hemiacetal is also proposed.



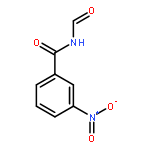

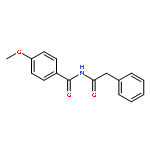

![Benzeneacetamide, N-[(4-methoxyphenyl)methyl]-](http://img.cochemist.com/ccimg/305900/305849-49-4.png)
![Benzeneacetamide, N-[(4-methoxyphenyl)methyl]-](http://img.cochemist.com/ccimg/305900/305849-49-4_b.png)
![4-Phenyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]-pyridine-6-carboxylic acid](http://img.cochemist.com/ccimg/178500/178456-18-3.png)
![4-Phenyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]-pyridine-6-carboxylic acid](http://img.cochemist.com/ccimg/178500/178456-18-3_b.png)
![4-phenyl-3H-Imidazo[4,5-c]pyridine](http://img.cochemist.com/ccimg/148800/148720-63-2.png)
![4-phenyl-3H-Imidazo[4,5-c]pyridine](http://img.cochemist.com/ccimg/148800/148720-63-2_b.png)






![Benzamide, N-[(4-methoxyphenyl)methyl]-4-nitro-](http://img.cochemist.com/ccimg/107700/107680-88-6.png)
![Benzamide, N-[(4-methoxyphenyl)methyl]-4-nitro-](http://img.cochemist.com/ccimg/107700/107680-88-6_b.png)