Co-reporter:L Feng, D Zhang, C Fan, C Ma, W Yang, Y Meng, W Wu, S Guan, B Jiang, M Yang, X Liu and D Guo
Cell Death & Disease 2013 4(7) pp:e715
Publication Date(Web):2013-07-01
DOI:10.1038/cddis.2013.222
HeLa cells treated with celastrol, a natural compound with inhibitive effect on proteasome, exhibited increase in apoptotic rate and characteristics of apoptosis. To clarify the signal network activated by celastrol to induce apoptosis, both the direct target proteins and undirect target proteins of celastrol were searched in the present study. Proteasome catalytic subunit β1 was predicted by computational analysis to be a possible direct target of celastrol and confirmed by checking direct effect of celastrol on the activity of recombinant human proteasome subunit β1 in vitro. Undirect target-related proteins of celastrol were searched using proteomic studies including two-dimensional electrophoresis (2-DE) analysis and iTRAQ-based LC-MS analysis. Possible target-related proteins of celastrol such as endoplasmic reticulum protein 29 (ERP29) and mitochondrial import receptor Tom22 (TOM22) were found by 2-DE analysis of total cellular protein expression profiles. Further study showed that celastrol induced ER stress and ER stress inhibitor could ameliorate cell death induced by celastrol. Celastrol induced translocation of Bax into the mitochondria, which might be related to the upregulation of BH-3-only proteins such as BIM and the increase in the expression level of TOM22. To further search possible target-related proteins of celastrol in ER and ER-related fractions, iTRAQ-based LC-MS method was use to analyze protein expression profiles of ER/microsomal vesicles-riched fraction of cells with or without celastrol treatment. Based on possible target-related proteins found in both 2-DE analysis and iTRAQ-based LC-MS analysis, protein–protein interaction (PPI) network was established using bioinformatic analysis. The important role of glycogen synthase kinase-3β (GSK3β) in the signal cascades of celastrol was suggested. Pretreatment of LiCL, an inhibitor of GSK3β, could significantly ameliorate apoptosis induced by celastrol. On the basis of the results of the present study, possible signal network of celastrol activated by celastrol leading to apoptosis was predicted.
Co-reporter:Lingjun Hu, Weibin Song, Yuhui Meng, Dean Guo, Xuan Liu, Lihong Hu
Bioorganic & Medicinal Chemistry Letters 2012 Volume 22(Issue 24) pp:7547-7550
Publication Date(Web):15 December 2012
DOI:10.1016/j.bmcl.2012.10.024
A series of 3-demethoxycarbonyl-3-acylamide methyl vinorelbine derivatives (compounds 7a–7z) were designed, synthesized, and evaluated for their inhibition activities against human non-small cell lung cancer cell line (A549). Most of the amide derivatives exhibited potent cytotoxicity, with the size of the introduced substituents being the foremost factor in determining the resultant cytotoxic activity. Test results in vivo against nude mice bearing A549 xenografts indicated that 7y showed comparable activities compared to the parent NVB.
Co-reporter:Weibin Song, Lingjun Hu, Yuhui Meng, Lei Ma, Dean Guo, Xuan Liu, Lihong Hu
Bioorganic & Medicinal Chemistry Letters 2012 Volume 22(Issue 10) pp:3485-3487
Publication Date(Web):15 May 2012
DOI:10.1016/j.bmcl.2012.03.082
A new series of vinorelbine analogues are designed and synthesized to explore the vindoline C-16 substituent effects on the biomimetic coupling with catharanthine, and the structure–activity relationships of these vinorelbine analogues as cytotoxic agents are also studied. The results show that introduction of severe steric hindrance and/or electron-withdrawing group at C-16 site are not propitious to improving the yields of the coupling reaction, and the SAR information collected so far suggests that small alkyl groups substituted at C-16 of vindoline are conductive to maintaining the cytotoxicity.
Co-reporter:Weibin Song, Min Lei, Kun Zhao, Lingjun Hu, Yuhui Meng, Dean Guo, Xuan Liu, Lihong Hu
Bioorganic & Medicinal Chemistry Letters 2012 Volume 22(Issue 1) pp:387-390
Publication Date(Web):1 January 2012
DOI:10.1016/j.bmcl.2011.10.114
A new, practical and efficient method for the synthesis of anhydrovinblastine AVBL (1f) by oxidative coupling of vindoline and catharanthine in the presence of ceric ammonium nitrate (CAN) was developed. Under the optimized reaction conditions, we synthesized a new series of amide anhydrovinblastine analogs substituted at the vindoline moiety of C-23 site and, evaluated for their proliferation inhibition against HeLa cell. The aryl-substituted derivatives showed loss of potency, while alkyl-substituted derivatives retained some of its cytotoxic potency. The iso-butylamide compound 10b and 2-furancorboxamide compound 18b displayed a similar cytotoxic potency compared to the positive control AVBL.
Co-reporter:Biao Ma, Zhi-Yong Xiao, Yi-Jia Chen, Min Lei, Yu-Hui Meng, De-An Guo, Xuan Liu, Li-Hong Hu
Steroids (May 2013) Volume 78(Issue 5) pp:508-512
Publication Date(Web):1 May 2013
DOI:10.1016/j.steroids.2013.02.007
A series of bufalin 3-nitrogen-containing-ester derivatives (2–6) were designed, synthesized, and evaluated for their proliferation inhibition activities against human cervical epithelial adenocarcinoma (HeLa) and non-small-cell lung cancer (A549) cell lines. The structure–activity relationships (SARs) of this new series were described in this paper. Cytotoxicity data revealed that C3 moiety had important influence on cytotoxic activity. On two cell lines, the bufalin-3-piperidinyl-4-carboxylate compound 2 (IC50 values on HeLa and A549 cell lines were 0.76 nM and 0.34 nM, respectively) displayed a significant cytotoxic potency compared to the parent compound bufalin.Graphical abstractDownload full-size imageHighlights► Two-step and highly efficient method to synthesize bufalin-3-ester derivatives. ► Bufalin 3-nitrogen-containing-ester derivatives show significant cytotoxicity. ► The cytotoxicity is correlated with the C3 moiety of bufalin.




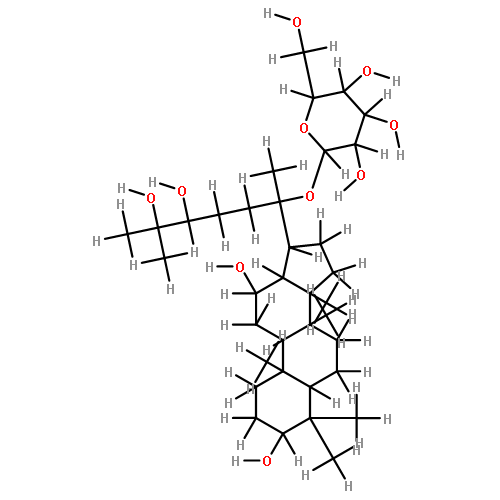


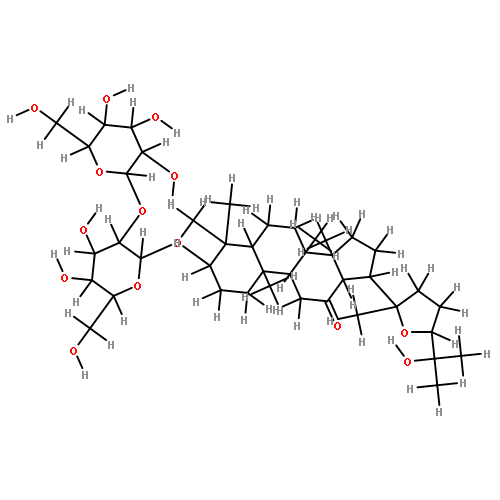
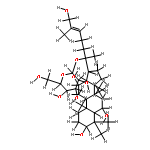
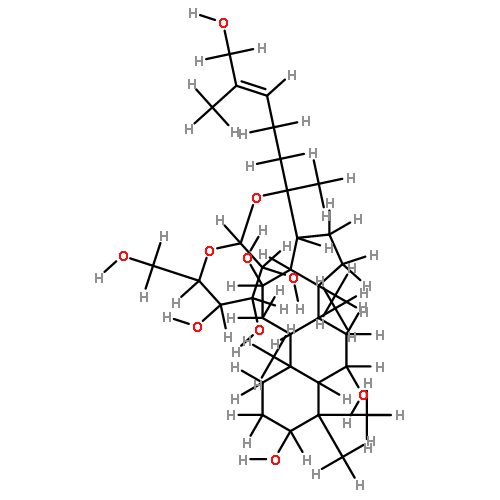
![6-O-beta-D-glucopyranosyl-20-O-[alpha-L-arabinofuranosyl-(1->6)-beta-D-glucopyranosyl]-dammar-24-ene-3beta,6alpha,12beta,20S-tetraol](http://img.cochemist.com/ccimg/1255300/1255210-79-7.png)
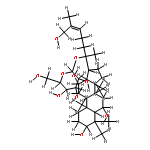
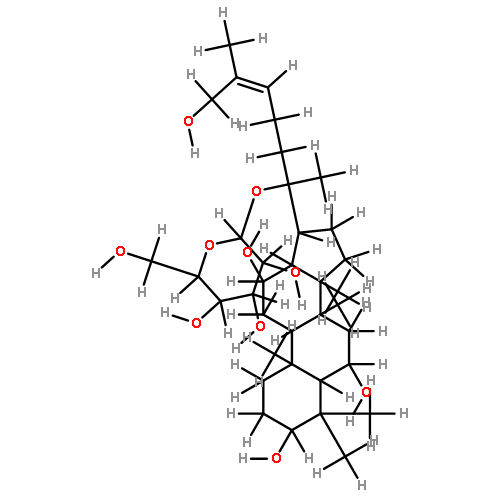
![6-O-beta-D-glucopyranosyl-20-O-[alpha-L-arabinofuranosyl-(1->6)-beta-D-glucopyranosyl]-dammar-24-ene-3beta,6alpha,12beta,20S-tetraol](http://img.cochemist.com/ccimg/1255300/1255210-79-7_b.png)