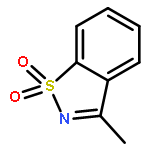
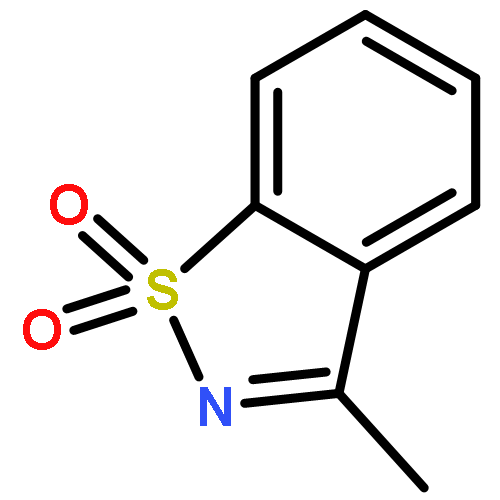
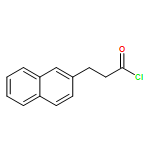
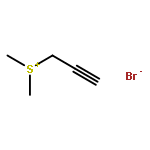
Co-reporter:Penghao Jia, Qinglong Zhang, Qima Ou, and You Huang
Organic Letters September 1, 2017 Volume 19(Issue 17) pp:
Publication Date(Web):August 22, 2017
DOI:10.1021/acs.orglett.7b02298
A powerful sequential [1 + 4]- and [2 + 3]-annulation has been developed using prop-2-ynylsulfonium salts and sulfonyl-protected o-amino aromatic aldimines, affording a series of hexahydropyrrolo[3,2-b]indoles in high yields. Prop-2-ynylsulfonium salts act as C2 synthons in the reaction, and fused rings containing two five-membered azaheterocycles can be constructed in a single operation with readily accessible starting materials.
Co-reporter:Penghao Jia;Qinglong Zhang;Hongxing Jin
Organic Letters January 20, 2017 Volume 19(Issue 2) pp:412-415
Publication Date(Web):December 29, 2016
DOI:10.1021/acs.orglett.6b03667
An unprecedented [3 + 2]-annulation of prop-2-ynylsulfonium salts and p-quinamines was developed, affording a series of hydroindol-5-ones with a methylthio group in moderate to good yields under mild conditions. In this reaction, the prop-2-ynylsulfonium salt acts as a novel C2 synthon and sulfide does not serve as a leaving group, which provides facile access to organosulfur compouds.
Co-reporter:Qinglong Zhang;Yannan Zhu;Hongxing Jin
Chemical Communications 2017 vol. 53(Issue 28) pp:3974-3977
Publication Date(Web):2017/04/04
DOI:10.1039/C6CC10155K
A novel phosphine mediated sequential annulation process to construct functionalized aza-benzobicyclo[4.3.0] derivatives has been developed involving a one-pot sequential catalytic and stoichiometric process, which generates a series of benzobicyclo[4.3.0] compounds containing one quaternary center with up to 94% yield and 20 : 1 dr value. In this reaction, MBH carbonates act as 1,2,3-C3 synthons.
Co-reporter:Hongxing Jin;Qinglong Zhang;Erqing Li;Penghao Jia;Ning Li
Organic & Biomolecular Chemistry 2017 vol. 15(Issue 34) pp:7097-7101
Publication Date(Web):2017/08/30
DOI:10.1039/C7OB01820G
A highly enantioselective intramolecular Rauhut–Currier reaction catalyzed by a multifunctional chiral aminophosphine catalyst was reported. A series of hydro-2H-indole derivatives that bear an all-carbon quaternary center were obtained in high yields (up to 94%), and excellent diastereo- and enantioselectivities (up to >20 : 1 dr and >99% ee). And this reaction could be performed on a gram scale using 2 mol% catalyst loading.
Co-reporter:Hongxing Jin;Erqing Li
Organic Chemistry Frontiers 2017 vol. 4(Issue 11) pp:2216-2220
Publication Date(Web):2017/10/24
DOI:10.1039/C7QO00596B
A nitrogen-containing Lewis base mediated regioselective [4 + 2] cyclization strategy between allenic esters and α,β-unsaturated ketimines was discovered, affording a series of hydropyridine derivatives in moderate to good yields under mild conditions.
Co-reporter:Penghao Jia and You Huang
Organic Letters 2016 Volume 18(Issue 10) pp:2475-2478
Publication Date(Web):May 12, 2016
DOI:10.1021/acs.orglett.6b01045
A sequential annulation domino reaction of sulfur ylides and α,β-unsaturated cyclic ketimines for the construction of cyclic 2-alkenyl aziridines has been developed. Readily accessible starting materials, a one-pot procedure, excellent functional group compatibility, and mild conditions make this transformation a powerful tool for the synthesis of cyclic 2-alkenyl aziridines.
Co-reporter:Ling Liang and You Huang
Organic Letters 2016 Volume 18(Issue 11) pp:2604-2607
Publication Date(Web):May 24, 2016
DOI:10.1021/acs.orglett.6b00988
The first phosphine-catalyzed [3 + 3]-domino annulation reaction of ynones and azomethine imines has been developed. With this simple and efficient method, the functionalized hydropyridazine derivatives were obtained in good to excellent yields with highly stereoselectivies.
Co-reporter:Meijia Chang;Chengzhou Wu;Jie Zheng; You Huang
Chemistry – An Asian Journal 2016 Volume 11( Issue 10) pp:1512-1517
Publication Date(Web):
DOI:10.1002/asia.201600102
Abstract
A new strategy for the one-pot synthesis of polysubstituted benzenes through a N,N-dimethyl-4-aminopyridine (DMAP)-catalyzed [4+2] benzannulation from readily prepared 1,3-bis(sulfonyl)butadienes and γ-substituted allenoates is described. This method provides a facile, metal-free and general route to highly substituted benzenes under mild conditions in moderate-to-good yields with complete regioselectivity.
Co-reporter:Ling Liang, Erqing Li, Xuelin Dong, and You Huang
Organic Letters 2015 Volume 17(Issue 19) pp:4914-4917
Publication Date(Web):September 23, 2015
DOI:10.1021/acs.orglett.5b02498
DABCO-mediated [4 + 4] domino annulation reactions of ynones and α-cyano-α,β-unsaturated ketones were discovered. The domino process affords an alternative route to eight-membered cyclic ethers in good yields under mild conditions.
Co-reporter:Peizhong Xie and You Huang
Organic & Biomolecular Chemistry 2015 vol. 13(Issue 32) pp:8578-8595
Publication Date(Web):18 Jun 2015
DOI:10.1039/C5OB00865D
Lewis base-promoted annulation reactions with MBHADs have emerged as a key platform for the construction of functionalized carbo- and heterocycles. MBHADs, which are economical and readily available, exert diverse and amazing reactivity when reacted with a wide range of electrophiles. A variety of carbo- and heterocycles, most of which are predominant in natural products and pharmaceuticals, could be constructed with high efficiency. This tutorial review will describe these annulation reactions, with a special emphasis on recent work regarding diverse reactivities of MBHADs.
Co-reporter:Erqing Li;Meijia Chang;Ling Liang
European Journal of Organic Chemistry 2015 Volume 2015( Issue 4) pp:710-714
Publication Date(Web):
DOI:10.1002/ejoc.201403369
Abstract
A strategy was developed to construct dihydropyran and multifunctional cyclopentene derivatives from the same starting materials by using different phosphine catalysts. By exploiting the different nucleophilicities of the phosphine catalysts, the γ-substituted allenoates selectively acted as C3 or C2 synthons. Under the catalysis of different organophosphine catalysts, the domino reaction proceeded smoothly with broad substrate tolerance and excellent total yields. The results suggest that the ethyl group of the γ-substituted allenoates played a key role in the domino reaction.
Co-reporter:Xuelin Dong;Ling Liang;Erqing Li ;Dr. You Huang
Angewandte Chemie International Edition 2015 Volume 54( Issue 5) pp:1621-1624
Publication Date(Web):
DOI:10.1002/anie.201409744
Abstract
The highly enantioselective intermolecular cross Rauhut–Currier reaction of different active olefins catalyzed by a multifunctional chiral Lewis base was reported. The RC products were obtained in excellent yields (up to 98 %), high chemo- and enantioselectivity (up to 96 % ee). The reaction could be performed on a gram scale using 1 mol % of the multifunctional phosphine catalyst.
Co-reporter:Xuelin Dong;Ling Liang;Erqing Li ;Dr. You Huang
Angewandte Chemie 2015 Volume 127( Issue 5) pp:1641-1644
Publication Date(Web):
DOI:10.1002/ange.201409744
Abstract
The highly enantioselective intermolecular cross Rauhut–Currier reaction of different active olefins catalyzed by a multifunctional chiral Lewis base was reported. The RC products were obtained in excellent yields (up to 98 %), high chemo- and enantioselectivity (up to 96 % ee). The reaction could be performed on a gram scale using 1 mol % of the multifunctional phosphine catalyst.
Co-reporter:Erqing Li, Penghao Jia, Ling Liang, and You Huang
ACS Catalysis 2014 Volume 4(Issue 2) pp:600
Publication Date(Web):January 10, 2014
DOI:10.1021/cs401161q
A novel phosphine-catalyzed sequential [2 + 3] and [3 + 2] annulation domino reaction of γ-benzyl-substituted allenoates has been developed. The reaction can proceed smoothly to produce the corresponding aza-bicyclo[3,3,0]octane derivatives in good yields and excellent diastereoselectivity (only one isomer).Keywords: domino reaction; ketimine; phosphine; sequential annulation; γ-substituent allenoates
Co-reporter:Jie Zheng, You Huang and Zhengming Li
Chemical Communications 2014 vol. 50(Issue 43) pp:5710-5713
Publication Date(Web):03 Apr 2014
DOI:10.1039/C4CC01097C
A convenient and efficient phosphine-catalyzed sequential annulation domino reaction between dienic sulfones and MBH carbonates has been developed. In the presence of 20 mol% of tris(4-fluorophenyl)phosphine, functionalized bicyclo[4.1.0]heptenes were prepared in excellent yields and stereoselectivities under mild conditions.
Co-reporter:Erqing Li and You Huang
Chemical Communications 2014 vol. 50(Issue 8) pp:948-950
Publication Date(Web):15 Nov 2013
DOI:10.1039/C3CC47716A
We have successfully developed a novel and efficient phosphine-catalyzed sequential [2+3] and [3+2] annulation reaction of γ-substituent allenoates to construct bicyclic[3, 3, 0]octene derivatives. The protocol uses readily available γ-substituent allenoates as the starting materials, inexpensive PBu3 as the catalyst, and the corresponding products were obtained in good to excellent yields under mild conditions.
Co-reporter:Fei Gao
Advanced Synthesis & Catalysis 2014 Volume 356( Issue 11-12) pp:2422-2428
Publication Date(Web):
DOI:10.1002/adsc.201400176
Co-reporter:Peizhong Xie;Jie Yang;Jie Zheng
European Journal of Organic Chemistry 2014 Volume 2014( Issue 6) pp:1189-1194
Publication Date(Web):
DOI:10.1002/ejoc.201301872
Abstract
Sequential catalysis of a [2+4] reaction between a Baylis–Hillman carbonate and a β,γ-unsaturated α-oxo ester for the synthesis of 2-methyl-2H-pyran was developed. The use of a Morita–Baylis–Hillman carbonate as a C2 synthon is first disclosed in this reaction. This method offers a new approach to the construction of 2-methyl-2H-pyrans and 2-oxabicyclo[2.2.2]oct-5-ene skeletons.
Co-reporter:Ling Liang;Erqing Li;Peizhong Xie ;Dr. You Huang
Chemistry – An Asian Journal 2014 Volume 9( Issue 5) pp:1270-1273
Publication Date(Web):
DOI:10.1002/asia.201301641
Abstract
An efficient synthetic approach has been developed for the construction of the spirocyclopentanone skeleton via a phosphine-catalyzed [3+2] annulation reaction. With this novel and economical protocol, various quaternary carbon-centered spirocyclopentanones could be readily obtained.
Co-reporter:Erqing Li ;Dr. You Huang
Chemistry - A European Journal 2014 Volume 20( Issue 12) pp:3520-3527
Publication Date(Web):
DOI:10.1002/chem.201304003
Abstract
A novel strategy that involves phosphine-catalyzed sequential [2+3] and [3+2] annulation reactions was developed. In this domino reaction, γ-substituted allenoates were used as novel C4 synthons, and the bicyclic cyclopenta[b]dihydrofuran derivatives were produced in good to excellent diastereoselectivities and yields under mild conditions. Furthermore, preliminary studies on an asymmetric variant of this reaction proceeded with moderate enantioselectivity.
Co-reporter:Da-Zhen Xu, Ming-Zhe Zhan, You Huang
Tetrahedron 2014 70(2) pp: 176-180
Publication Date(Web):
DOI:10.1016/j.tet.2013.11.103
Co-reporter:Hongxia Zhao, Xiangtai Meng and You Huang
Chemical Communications 2013 vol. 49(Issue 89) pp:10513-10515
Publication Date(Web):23 Sep 2013
DOI:10.1039/C3CC46379F
PPh3-Catalyzed aza-MBH domino reaction of salicyl N-tosylimines with γ-CH3 substituted allenoates is reported. Readily available imines and allenoates are converted to benzoxazepine derivatives in one step. Wherein, functionalization of C–H bonds of γ-substituted allenoate has been developed in this domino process.
Co-reporter:Erqing Li, You Huang, Ling Liang, and Peizhong Xie
Organic Letters 2013 Volume 15(Issue 12) pp:3138-3141
Publication Date(Web):June 6, 2013
DOI:10.1021/ol401249e
The first phosphine-catalyzed [4 + 2] annulation of γ-substituted allenoates with 2-arylidene-1H-indene-1,3(2H)-diones is disclosed. In the reaction, the γ-substituted allenoate serves as a new type of 1,4-dipolar synthon; this broadens the application of γ-substituted allenoates. This method also offers a powerful approach to the construction of highly substituted spiro[4.5]dec-6-ene skeletons in excellent yields, and with complete regioselectivity and high diastereoselectivity.
Co-reporter:Jie Zheng, You Huang, and Zhengming Li
Organic Letters 2013 Volume 15(Issue 19) pp:5064-5067
Publication Date(Web):September 13, 2013
DOI:10.1021/ol4022904
The first phosphine-catalyzed domino benzannulation reaction to prepare a variety of functionalized biaryls from allenoates and dienic sulfones is developed.
Co-reporter:Jie Zheng, You Huang, and Zhengming Li
Organic Letters 2013 Volume 15(Issue 22) pp:5758-5761
Publication Date(Web):October 31, 2013
DOI:10.1021/ol402799w
Dienic sulfones and γ-CH3 allenoates can be converted into bicyclo[3.2.0]heptene derivatives in moderate to good yield using trimethylphosphine as the catalyst under mild conditions.
Co-reporter:Peizhong Xie;Erqing Li;Jie Zheng;Xin Li;Ruyu Chen
Advanced Synthesis & Catalysis 2013 Volume 355( Issue 1) pp:161-169
Publication Date(Web):
DOI:10.1002/adsc.201200675
Abstract
We have developed a novel strategy to control the product distribution between 2,3-dihydrofurans and biaryls from the same starting materials by tuning the catalytic or stoichiometric process. By controlling the loading of the phosphine PR3, the Morita–Baylis–Hillman carbonates can be selectively used as a C1 or a C3 synthon, respectively. This investigation has given new insights into tunable domino reactions and will be useful in diversity-oriented synthesis (DOS).
Co-reporter:Peizhong Xie
European Journal of Organic Chemistry 2013 Volume 2013( Issue 28) pp:6213-6226
Publication Date(Web):
DOI:10.1002/ejoc.201300469
Abstract
The Rauhut–Currier reaction has proved itself as a promising strategy for synthesis of multifunctionalized molecules, although still being in its infancy with regard to limitations of selectivity and scope. Considerable attention has thus been devoted to resolution of difficulties associated with controlling strategies and enantioselectivity. Carbocycles with distinct structural frameworks can be assembled rapidly through incorporation of cross-Rauhut–Currier reactions into domino processes. The fine compatibility and widespread utility of Rauhut–Currier reactions is illustrated with several recent outstanding examples, with the aim of shedding light on the design of new substrates and catalysts for syntheses of complex molecules.
Co-reporter:Erqing Li;Peizhong Xie;Lihua Yang;Ling Liang ; You Huang
Chemistry – An Asian Journal 2013 Volume 8( Issue 3) pp:603-610
Publication Date(Web):
DOI:10.1002/asia.201201087
Abstract
A new tunable phosphine-catalyzed aza-Michael β-addition reaction between allenoates and various hydrazones has been developed. These reactions are most-efficiently promoted by a catalytic amount of phosphine catalysts. These atom-economical reactions are operationally simple and their corresponding adducts can been achieved in high yields and high selectivity under mild reaction conditions. Further studies revealed that different phosphine catalyst can produce different adducts from the same starting materials.
Co-reporter:Chongchong Hu;Qinglong Zhang ;Dr. You Huang
Chemistry – An Asian Journal 2013 Volume 8( Issue 9) pp:1981-1984
Publication Date(Web):
DOI:10.1002/asia.201300650
Co-reporter:Lihua Yang, Peizhong Xie, Erqing Li, Xin Li, You Huang and Ruyu Chen
Organic & Biomolecular Chemistry 2012 vol. 10(Issue 37) pp:7628-7634
Publication Date(Web):02 Aug 2012
DOI:10.1039/C2OB26338F
A novel phosphine-catalyzed intermolecular [3 + 2] cycloaddition of ynones and N-substituted isatins was developed. In this reaction, substituted ynones, serving as a C3 synthon, were successfully applied in intermolecular annulation reactions. A number of functionalized spirooxazolines were obtained in high yields and stereoselectivity.
Co-reporter:Xiangtai Meng, Peizhong Xie, You Huang and Ruyu Chen
RSC Advances 2012 vol. 2(Issue 21) pp:8104-8109
Publication Date(Web):27 Jun 2012
DOI:10.1039/C2RA20823G
We report two domino reactions between salicyl N-thiophosphoryl imines and methyl vinyl ketone catalyzed by bifunctional phosphine catalysts and DBU. These reactions provide two simple and efficient methods to construct chromans and chromens under mild conditions, respectively.
Co-reporter:Peizhong Xie;Dr. You Huang;Dr. Ruyu Chen
Chemistry - A European Journal 2012 Volume 18( Issue 24) pp:7362-7366
Publication Date(Web):
DOI:10.1002/chem.201200305
Co-reporter:Chongchong Hu;Zhishuai Geng;Jianze Ma;Dr. You Huang;Dr. Ruyu Chen
Chemistry – An Asian Journal 2012 Volume 7( Issue 9) pp:2032-2035
Publication Date(Web):
DOI:10.1002/asia.201200308
Co-reporter:Peizhong Xie;Wenqing Lai;Zhishuai Geng;Dr. You Huang;Dr. Ruyu Chen
Chemistry – An Asian Journal 2012 Volume 7( Issue 7) pp:1533-1537
Publication Date(Web):
DOI:10.1002/asia.201200140
Co-reporter:Jianze Ma, You Huang and Ruyu Chen
Organic & Biomolecular Chemistry 2011 vol. 9(Issue 6) pp:1791-1798
Publication Date(Web):30 Nov 2010
DOI:10.1039/C0OB00725K
A novel NHC-catalyzed three-component domino strategy to access high functionalized cis-ε-ketoesters with excellent yields (up to 98%) and high stereoselectivities (up to 20:1) is documented. The title domino reactions are atom economical and work on a broad range of substrates. The relative stereochemistry could be explained by a cascade crossed-benzoin/oxy-Cope rearrangement/esterification process. The thus-obtained products are of potential synthetic value in the drug research and combinatorial chemistry.
Co-reporter:Peizhong Xie, You Huang, Wenqing Lai, Xiangtai Meng and Ruyu Chen
Organic & Biomolecular Chemistry 2011 vol. 9(Issue 19) pp:6707-6714
Publication Date(Web):23 Jun 2011
DOI:10.1039/C1OB05693J
A novel bifunctional phosphine-catalyzed reaction was developed. Cross-Rauhut–Currier, Michael and aldol reactions were successfully combined into a domino process. This method offers a powerful approach to the construction of highly substituted cyclohexene skeletons.
Co-reporter:Peizhong Xie, Liye Wang, Lihua Yang, Erqing Li, Jianze, Ma, You Huang, and Ruyu Chen
The Journal of Organic Chemistry 2011 Volume 76(Issue 19) pp:7699-7705
Publication Date(Web):August 11, 2011
DOI:10.1021/jo2008737
A novel domino annulation between sulfur ylides and salicyl N-thiophosphinyl imines was developed. The method allows the synthesis of a highly substituted trans-2,3-dihydrobenzofuran skeleton with high yield and excellent chemo- and stereoselectivity.
Co-reporter:Jianze Ma;Dr. Peizhong Xie;Chongchong Hu;Dr. You Huang;Dr. Ruyu Chen
Chemistry - A European Journal 2011 Volume 17( Issue 27) pp:7418-7422
Publication Date(Web):
DOI:10.1002/chem.201100881
Co-reporter:Peizhong Xie, You Huang and Ruyu Chen
Organic Letters 2010 Volume 12(Issue 17) pp:3768-3771
Publication Date(Web):August 12, 2010
DOI:10.1021/ol101611v
A novel phosphine-catalyzed domino reaction of salicyl N-thiophosphinyl imines and allylic carbonates was developed. The allylic carbonate, in this reaction, serves as a new kind of 1,1-dipolar synthon. This method offered a powerful approach to the construction of a highly substituted trans-2,3-dihydrobenzofuran skeleton with high diastereoselectivity.
Co-reporter:Xiangtai Meng Dr., Dr. ;Ruyu Chen Dr.
Chemistry - A European Journal 2008 Volume 14( Issue 23) pp:6852-6856
Publication Date(Web):
DOI:10.1002/chem.200800753
Co-reporter:Ling-yan Liu;Ru-yu Chen
Heteroatom Chemistry 2005 Volume 16(Issue 1) pp:33-38
Publication Date(Web):20 JAN 2005
DOI:10.1002/hc.20060
In order to search for novel antitumor and antiviral agents with high activity and low toxicity, a series of chiral 2-thio(oxo)-1,3,2-oxazaphospholidines were synthesized via the reaction of L-methionol with all kinds of (thio)phosphoryl dichlorides in THF in the presence of triethylamine at room temperature. The structures of all of the new compounds were confirmed by elemental analyses, 1H, 31P NMR, and IR spectra. © 2005 Wiley Periodicals, Inc. Heteroatom Chem 16:33–38, 2005; Published online in Wiley InterScience (www.interscience.wiley.com). DOI 10.1002/hc.20060
Co-reporter:Qinglong Zhang, Yannan Zhu, Hongxing Jin and You Huang
Chemical Communications 2017 - vol. 53(Issue 28) pp:NaN3977-3977
Publication Date(Web):2017/03/16
DOI:10.1039/C6CC10155K
A novel phosphine mediated sequential annulation process to construct functionalized aza-benzobicyclo[4.3.0] derivatives has been developed involving a one-pot sequential catalytic and stoichiometric process, which generates a series of benzobicyclo[4.3.0] compounds containing one quaternary center with up to 94% yield and 20:1 dr value. In this reaction, MBH carbonates act as 1,2,3-C3 synthons.
Co-reporter:Peizhong Xie, You Huang, Wenqing Lai, Xiangtai Meng and Ruyu Chen
Organic & Biomolecular Chemistry 2011 - vol. 9(Issue 19) pp:NaN6714-6714
Publication Date(Web):2011/06/23
DOI:10.1039/C1OB05693J
A novel bifunctional phosphine-catalyzed reaction was developed. Cross-Rauhut–Currier, Michael and aldol reactions were successfully combined into a domino process. This method offers a powerful approach to the construction of highly substituted cyclohexene skeletons.
Co-reporter:Peizhong Xie and You Huang
Organic & Biomolecular Chemistry 2015 - vol. 13(Issue 32) pp:NaN8595-8595
Publication Date(Web):2015/06/18
DOI:10.1039/C5OB00865D
Lewis base-promoted annulation reactions with MBHADs have emerged as a key platform for the construction of functionalized carbo- and heterocycles. MBHADs, which are economical and readily available, exert diverse and amazing reactivity when reacted with a wide range of electrophiles. A variety of carbo- and heterocycles, most of which are predominant in natural products and pharmaceuticals, could be constructed with high efficiency. This tutorial review will describe these annulation reactions, with a special emphasis on recent work regarding diverse reactivities of MBHADs.
Co-reporter:Jie Zheng, You Huang and Zhengming Li
Chemical Communications 2014 - vol. 50(Issue 43) pp:NaN5713-5713
Publication Date(Web):2014/04/03
DOI:10.1039/C4CC01097C
A convenient and efficient phosphine-catalyzed sequential annulation domino reaction between dienic sulfones and MBH carbonates has been developed. In the presence of 20 mol% of tris(4-fluorophenyl)phosphine, functionalized bicyclo[4.1.0]heptenes were prepared in excellent yields and stereoselectivities under mild conditions.
Co-reporter:Erqing Li and You Huang
Chemical Communications 2014 - vol. 50(Issue 8) pp:NaN950-950
Publication Date(Web):2013/11/15
DOI:10.1039/C3CC47716A
We have successfully developed a novel and efficient phosphine-catalyzed sequential [2+3] and [3+2] annulation reaction of γ-substituent allenoates to construct bicyclic[3, 3, 0]octene derivatives. The protocol uses readily available γ-substituent allenoates as the starting materials, inexpensive PBu3 as the catalyst, and the corresponding products were obtained in good to excellent yields under mild conditions.
Co-reporter:Hongxia Zhao, Xiangtai Meng and You Huang
Chemical Communications 2013 - vol. 49(Issue 89) pp:NaN10515-10515
Publication Date(Web):2013/09/23
DOI:10.1039/C3CC46379F
PPh3-Catalyzed aza-MBH domino reaction of salicyl N-tosylimines with γ-CH3 substituted allenoates is reported. Readily available imines and allenoates are converted to benzoxazepine derivatives in one step. Wherein, functionalization of C–H bonds of γ-substituted allenoate has been developed in this domino process.
Co-reporter:Lihua Yang, Peizhong Xie, Erqing Li, Xin Li, You Huang and Ruyu Chen
Organic & Biomolecular Chemistry 2012 - vol. 10(Issue 37) pp:NaN7634-7634
Publication Date(Web):2012/08/02
DOI:10.1039/C2OB26338F
A novel phosphine-catalyzed intermolecular [3 + 2] cycloaddition of ynones and N-substituted isatins was developed. In this reaction, substituted ynones, serving as a C3 synthon, were successfully applied in intermolecular annulation reactions. A number of functionalized spirooxazolines were obtained in high yields and stereoselectivity.
Co-reporter:Jianze Ma, You Huang and Ruyu Chen
Organic & Biomolecular Chemistry 2011 - vol. 9(Issue 6) pp:NaN1798-1798
Publication Date(Web):2010/11/30
DOI:10.1039/C0OB00725K
A novel NHC-catalyzed three-component domino strategy to access high functionalized cis-ε-ketoesters with excellent yields (up to 98%) and high stereoselectivities (up to 20:1) is documented. The title domino reactions are atom economical and work on a broad range of substrates. The relative stereochemistry could be explained by a cascade crossed-benzoin/oxy-Cope rearrangement/esterification process. The thus-obtained products are of potential synthetic value in the drug research and combinatorial chemistry.