Co-reporter:Luke J. Peterson, Jingyi Luo, and John P. Wolfe
Organic Letters June 2, 2017 Volume 19(Issue 11) pp:
Publication Date(Web):May 23, 2017
DOI:10.1021/acs.orglett.7b00946
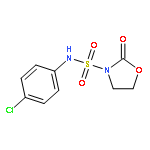
Palladium-catalyzed carboamination reactions of N-allylguanidines bearing cleavable N-cyano or N-arylsulfonyl protecting groups are described. The reactions afford cyclic guanidine products in good yield, and transformations of substrates bearing internal alkenes proceed with high diastereoselectivity. Deuterium labeling studies indicate these transformations proceed via anti-aminopalladation pathways.
Co-reporter:Jordan R. Boothe, Yifan Shen, and John P. Wolfe
The Journal of Organic Chemistry 2017 Volume 82(Issue 5) pp:
Publication Date(Web):February 23, 2017
DOI:10.1021/acs.joc.7b00041
The synthesis of substituted γ- and δ-lactams via palladium-catalyzed alkene carboamination reactions between aryl halides and alkenes bearing pendant amides is described. The substrates for these reactions are generated in 1–3 steps from commercially available materials, and products are obtained in good yield with up to >20:1 diastereoselectivity. The stereochemical outcome of the alkene addition is consistent with a mechanism involving anti-aminopalladation of the alkene.
Co-reporter:Zachary J. Garlets;Derick R. White; John P. Wolfe
Asian Journal of Organic Chemistry 2017 Volume 6(Issue 6) pp:636-653
Publication Date(Web):2017/06/01
DOI:10.1002/ajoc.201600577
AbstractThis Focus Review summarizes recent developments in palladium-catalyzed alkene carboalkoxylation and carboamination reactions. New synthetic methods that have been reported within the past four years are described, along with mechanistic insights and the influence of reaction mechanism on product stereochemistry. The applications of these transformations to the synthesis of natural products and other biologically relevant compounds are also discussed.
Co-reporter:Zachary J. Garlets, Mattia Silvi, and John P. Wolfe
Organic Letters 2016 Volume 18(Issue 10) pp:2331-2334
Publication Date(Web):May 11, 2016
DOI:10.1021/acs.orglett.6b00598
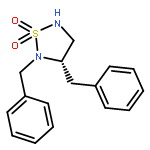
The silver-catalyzed hydroamination of tosyl-protected N-allylguanidines is described. These reactions provide substituted cyclic guanidines in high yields. The reactions are amenable to the construction of quaternary stereocenters as well as both monocyclic and bicyclic guanidine products.
Co-reporter:Zachary J. Garlets;Kaia R. Parenti ;Dr. John P. Wolfe
Chemistry - A European Journal 2016 Volume 22( Issue 17) pp:5919-5922
Publication Date(Web):
DOI:10.1002/chem.201600887
Abstract
The synthesis of cyclic sulfamides by enantioselective Pd-catalyzed alkene carboamination reactions between N-allylsulfamides and aryl or alkenyl bromides is described. High levels of asymmetric induction (up to 95:5 e.r.) are achieved using a catalyst composed of [Pd2(dba)3] and (S)-Siphos-PE. Deuterium-labelling studies indicate the reactions proceed through syn-aminopalladation of the alkene and suggest that the control of syn- versus anti-aminopalladation pathways is important for asymmetric induction.
Co-reporter:Derick R. White; Johnathon T. Hutt
Journal of the American Chemical Society 2015 Volume 137(Issue 35) pp:11246-11249
Publication Date(Web):August 27, 2015
DOI:10.1021/jacs.5b07203
A new type of Pd-catalyzed alkene carboamination reaction that provides direct access to enantioenriched 2-aminoindanes from 2-allylphenyltriflate derivatives and aliphatic amines is described. A catalyst generated in situ from Pd(OAc)2 and (S)-tert-butylPHOX provides the functionalized carbocycles in good yield with up to >99:1 er. The transformations occur via a key anti-aminopalladation that involves intermolecular attack of an amine nucleophile on an arylpalladium alkene complex.
Co-reporter:Derick R. White and John P. Wolfe
Organic Letters 2015 Volume 17(Issue 10) pp:2378-2381
Publication Date(Web):May 6, 2015
DOI:10.1021/acs.orglett.5b00896
Cascade Pd-catalyzed alkene carboamination/Diels–Alder reactions between bromodienes and amines bearing two pendant alkenes are described. These transformations generate 4 bonds, 3 rings, and 3–5 stereocenters to afford polycyclic nitrogen heterocycles with high diastereoselectivity.
Co-reporter:Luke J. Peterson
Advanced Synthesis & Catalysis 2015 Volume 357( Issue 10) pp:2339-2344
Publication Date(Web):
DOI:10.1002/adsc.201500334
Co-reporter:Renata K. Everett and John P. Wolfe
The Journal of Organic Chemistry 2015 Volume 80(Issue 18) pp:9041-9056
Publication Date(Web):September 1, 2015
DOI:10.1021/acs.joc.5b01286
Treatment of N-(arylmethyl)-N-aryl or N-allyl-N-aryl glycine methyl ester derivatives with nBu2BOTf and iPr2NEt effects an aza-Wittig rearrangement that provides N-aryl phenylalanine methyl ester derivatives and N-aryl allylglycine methyl ester derivatives, respectively, in good yield with moderate to good diastereoselectivity. Under similar conditions, analogous substrates bearing N-carbonyl groups are converted to 1,4,2-oxazaborole derivatives. Additionally, N-allyl-N-aryl glycine methyl ester derivatives subjected to similar conditions at elevated temperatures undergo an aza-[2,3]-Wittig rearrangement, followed by a subsequent hydroboration oxidation reaction, to afford substituted amino alcohol products.
Co-reporter:Brett A. Hopkins;Zachary J. Garlets ;Dr. John P. Wolfe
Angewandte Chemie International Edition 2015 Volume 54( Issue 45) pp:13390-13392
Publication Date(Web):
DOI:10.1002/anie.201506884
Abstract
The Pd-catalyzed coupling of γ-hydroxyalkenes with aryl bromides affords enantiomerically enriched 2-(arylmethyl)tetrahydrofuran derivatives in good yield and up to 96:4 e.r. This transformation was achieved through the development of a new TADDOL/2-arylcyclohexanol-derived chiral phosphite ligand. The transformations are effective with an array of different aryl bromides, and can be used for the preparation of products bearing quaternary stereocenters.
Co-reporter:Brett A. Hopkins;Zachary J. Garlets ;Dr. John P. Wolfe
Angewandte Chemie 2015 Volume 127( Issue 45) pp:13588-13590
Publication Date(Web):
DOI:10.1002/ange.201506884
Abstract
The Pd-catalyzed coupling of γ-hydroxyalkenes with aryl bromides affords enantiomerically enriched 2-(arylmethyl)tetrahydrofuran derivatives in good yield and up to 96:4 e.r. This transformation was achieved through the development of a new TADDOL/2-arylcyclohexanol-derived chiral phosphite ligand. The transformations are effective with an array of different aryl bromides, and can be used for the preparation of products bearing quaternary stereocenters.
Co-reporter:B. A. Hopkins and J. P. Wolfe
Chemical Science 2014 vol. 5(Issue 12) pp:4840-4844
Publication Date(Web):27 Aug 2014
DOI:10.1039/C4SC01327A
A catalyst composed of Pd2(dba)3 and (S)-Siphos-PE provides excellent results in Pd-catalyzed alkene carboamination reactions between aniline derivatives bearing pendant alkenes and aryl or alkenyl halides. These transformations generate tetrahydroquinolines and tetrahydroquinoxalines that contain quaternary carbon stereocenters with high levels of asymmetric induction. In addition this catalyst also effects the asymmetric synthesis of tetrahydroisoquinolines via related transformations of 2-allylbenzylamines. In contrast to most other approaches to the asymmetric synthesis of these compounds, which frequently involve functional group interconversion or a single C–C or C–N bond-forming event, the carboamination reactions generate both a C–N bond, a C–C bond, and a stereocenter.
Co-reporter:Nicholas R. Babij, Grace M. McKenna, Ryan M. Fornwald, and John P. Wolfe
Organic Letters 2014 Volume 16(Issue 12) pp:3412-3415
Publication Date(Web):June 11, 2014
DOI:10.1021/ol5015976
A new annulation strategy for the synthesis of trans-bicyclic sulfamides is described. The Pd-catalyzed alkene carboamination reactions of 2-allyl and cis-2,5-diallyl pyrrolidinyl sulfamides with aryl and alkenyl triflates afford the fused bicyclic compounds in good yields and with good diastereoselectivity (up to 13:1 dr). Importantly, by employing reaction conditions that favor an anti-aminopalladation mechanism, the relative stereochemistry between the C3 and C4a stereocenters of the products is reversed relative to related Pd-catalyzed carboamination reactions that proceed via syn-aminopalladation.
Co-reporter:Blane P. Zavesky, Nicholas R. Babij, and John P. Wolfe
Organic Letters 2014 Volume 16(Issue 18) pp:4952-4955
Publication Date(Web):September 8, 2014
DOI:10.1021/ol502471x
A new method for the synthesis of 2-aminoimidazole products is described. The heterocyclic products are generated in good yields via Pd-catalyzed carboamination reactions of N-propargyl guanidines and aryl triflates. This methodology generates both a C–N and C–C bond during the annulation step and facilitates the rapid construction of 2-aminoimidazole products with different aryl groups. The utility of this methodology was demonstrated in the total synthesis of preclathridine A, preclathridine B, and dorimidazole B from a single intermediate.
Co-reporter:Ryan M. Fornwald;Dr. Jonathan A. Fritz;Dr. John P. Wolfe
Chemistry - A European Journal 2014 Volume 20( Issue 28) pp:8782-8790
Publication Date(Web):
DOI:10.1002/chem.201402258
Abstract
The Pd-catalyzed coupling of N-allylsulfamides with aryl and alkenyl triflates to afford cyclic sulfamide products is described. In contrast to other known Pd-catalyzed alkene carboamination reactions, these transformations may be selectively induced to occur by way of either anti- or syn-aminopalladation mechanistic pathways by modifying the catalyst structure and reaction conditions.
Co-reporter:Jeremiah Alicea and John P. Wolfe
The Journal of Organic Chemistry 2014 Volume 79(Issue 9) pp:4212-4217
Publication Date(Web):April 11, 2014
DOI:10.1021/jo500470m
Intramolecular Pd-catalyzed alkene carboamination reactions of substituted 2-allyl-N-(2-bromobenzyl)anilines are described. The substrates for these reactions are generated in two steps from readily available 2-allylanilines and 2-bromobenzaldehyde derivatives. The transformations afford substituted tetrahydroindoloisoquinolines, an uncommon class of fused bicyclic heterocycles, in good yield. The mechanism of these transformations is described, and a model that accounts for the observed product stereochemistry is proposed.
Co-reporter:Blane P. Zavesky, Nicholas R. Babij, Jonathan A. Fritz, and John P. Wolfe
Organic Letters 2013 Volume 15(Issue 21) pp:5420-5423
Publication Date(Web):October 22, 2013
DOI:10.1021/ol402377y
A new approach to the synthesis of substituted 5-membered cyclic guanidines is described. Palladium-catalyzed alkene carboamination reactions between acyclic N-allyl guanidines and aryl or alkenyl halides provide these products in good yield. This method allows access to a number of different cyclic guanidine derivatives in only two steps from readily available allylic amines.
Co-reporter:Renata K. Everett and John P. Wolfe
Organic Letters 2013 Volume 15(Issue 12) pp:2926-2929
Publication Date(Web):June 11, 2013
DOI:10.1021/ol4009188
Treatment of methyl O-(alkynylmethyl) glycolate derivatives with dialkylboron triflates and Hünig’s base leads to the formation of highly substituted 3-hydroxy-2-furanone derivatives. The transformations appear to proceed via an unusual mechanism involving initial 2,3-Wittig rearrangement of a boron ester enolate followed by an alkylative cyclization reaction that leads to incorporation of an alkyl group from the boron reagent into the product.
Co-reporter:Nicholas R. Babij ; John P. Wolfe
Angewandte Chemie International Edition 2013 Volume 52( Issue 35) pp:9247-9250
Publication Date(Web):
DOI:10.1002/anie.201302720
Co-reporter:Nicholas R. Babij ; John P. Wolfe
Angewandte Chemie 2013 Volume 125( Issue 35) pp:9417-9420
Publication Date(Web):
DOI:10.1002/ange.201302720
Co-reporter:Amanda F. Ward, Yan Xu and John P. Wolfe
Chemical Communications 2012 vol. 48(Issue 4) pp:609-611
Publication Date(Web):24 Nov 2011
DOI:10.1039/C1CC15880E
A new method for the construction of 2-substituted and 2,2-disubstituted chromans viaPd-catalyzed carboetherification reactions between aryl/alkenyl halides and 2-(but-3-en-1-yl)phenols is described. These reactions effect formation of a C–O bond and a C–C bond to afford the chroman products in good yield, and also provide stereoselective access to tricyclic chroman derivatives.
Co-reporter:Danielle M. Schultz;Nicholas R. Babij
Advanced Synthesis & Catalysis 2012 Volume 354( Issue 18) pp:3451-3455
Publication Date(Web):
DOI:10.1002/adsc.201200825
Abstract
A new gold(I)-catalyzed multicomponent synthesis of β-alkoxy ketones from aldehydes, alcohols, and alkynes is described. This atom economical synthesis was achieved through the use of the gold complex (SPhos)AuNTf2 as a catalyst, and allows for the preparation of a diverse array of β-alkoxy ketone products. Mechanistic studies illustrate that these reactions proceed via gold(I)-catalyzed hydrolysis of the alkyne to an aryl ketone, which then undergoes an aldol reaction with an oxocarbenium ion generated in situ from the aldehyde and alcohol components.
Co-reporter:Brett A. Hopkins ;Dr. John P. Wolfe
Angewandte Chemie International Edition 2012 Volume 51( Issue 39) pp:9886-9890
Publication Date(Web):
DOI:10.1002/anie.201205233
Co-reporter:Brett A. Hopkins ;Dr. John P. Wolfe
Angewandte Chemie 2012 Volume 124( Issue 39) pp:10024-10028
Publication Date(Web):
DOI:10.1002/ange.201205233
Co-reporter:Dr. John P. Wolfe
Angewandte Chemie 2012 Volume 124( Issue 41) pp:10370-10371
Publication Date(Web):
DOI:10.1002/ange.201204470
Co-reporter:Nicholas R. Babij ; John P. Wolfe
Angewandte Chemie 2012 Volume 124( Issue 17) pp:4204-4206
Publication Date(Web):
DOI:10.1002/ange.201201001
Co-reporter:Nicholas R. Babij ; John P. Wolfe
Angewandte Chemie International Edition 2012 Volume 51( Issue 17) pp:4128-4130
Publication Date(Web):
DOI:10.1002/anie.201201001
Co-reporter:Dr. John P. Wolfe
Angewandte Chemie International Edition 2012 Volume 51( Issue 41) pp:10224-10225
Publication Date(Web):
DOI:10.1002/anie.201204470
Co-reporter:Joshua D. Neukom, Alvin S. Aquino, and John P. Wolfe
Organic Letters 2011 Volume 13(Issue 9) pp:2196-2199
Publication Date(Web):March 29, 2011
DOI:10.1021/ol200429a
A new synthesis of 1,4-benzodiazepines and 1,4-benzodiazepin-5-ones is reported. The Pd-catalyzed coupling of N-allyl-2-aminobenzylamine derivatives with aryl bromides affords the heterocyclic products in good yield, and substrates bearing allylic methyl groups are transformed to cis-2,3-disubstituted products with >20:1 dr.
Co-reporter:Danielle M. Schultz and John P. Wolfe
Organic Letters 2011 Volume 13(Issue 11) pp:2962-2965
Publication Date(Web):May 11, 2011
DOI:10.1021/ol201051q
The synthesis of tropane derivatives via intramolecular Pd-catalyzed alkene difunctionalization reactions is described. Enantiopure N-aryl-γ-aminoalkenes bearing an aryl or alkenyl halide adjacent to the amino group were converted to benzo- or cycloalkenyl-fused tropane products in good yield and with no loss of enantiopurity.
Co-reporter:Duy N. Mai, Brandon R. Rosen, and John P. Wolfe
Organic Letters 2011 Volume 13(Issue 11) pp:2932-2935
Publication Date(Web):May 10, 2011
DOI:10.1021/ol2009895
A concise asymmetric synthesis of (+)-aphanorphine has been achieved via a new enantioconvergent strategy. A racemic γ-aminoalkene derivative is transformed into a 1:1 mixture of enantiomerically enriched diastereomers using an asymmetric Pd-catalyzed carboamination. This mixture is then converted to an enantiomerically enriched protected aphanorphine derivative by a Friedel–Crafts reaction, which generates a quaternary all-carbon stereocenter. The natural product is obtained in three additional steps.
Co-reporter:Joshua D. Neukom, Nicholas S. Perch, and John P. Wolfe
Organometallics 2011 Volume 30(Issue 5) pp:1269-1277
Publication Date(Web):February 3, 2011
DOI:10.1021/om200008t
Studies on the synthesis and reactivity of a series of (P−P)Pd(Ar)[N(Ar1)(CH2)3CR═CHR′] complexes 3 are described. These complexes are transformed to observable (P−P)Pd(Ar)[pyrrolidin-2-ylmethyl] complexes 4 via syn insertion of the pendant alkene into the Pd−N bond. Complexes 4 then undergo C−C bond-forming reductive elimination to yield N-aryl-2-benzylpyrrolidine derivatives 2. Kinetic studies indicate the rates of conversion of 3 to 4 and 4 to 2 are within 1 order of magnitude. The effects of phosphine ligand structure, alkene substitution, and the electronic properties of the Ar and Ar1 groups on reaction rates are reported, as are the results of deuterium isotope effect studies. The mechanism of the aminopalladation step is discussed in detail, and the results of the experiments described in this paper are most consistent with conversion of 3 to 4 via rate-determining ligand displacement followed by fast aminopalladation. These transformations represent rare examples of syn migratory insertion of unactivated alkenes into Pd−N bonds.
Co-reporter:Duy N. Mai
Journal of the American Chemical Society 2010 Volume 132(Issue 35) pp:12157-12159
Publication Date(Web):August 18, 2010
DOI:10.1021/ja106989h
The enantioselective synthesis of 2-(arylmethyl)- and 2-(alkenylmethyl)pyrrolidine derivatives via Pd-catalyzed alkene carboamination reactions is described. These transformations generate enantiomerically enriched products with up to 94% ee from readily available alkenyl or aryl bromides and N-boc-pent-4-enylamines. The application of this method to a concise asymmetric synthesis of (−)-tylophorine is also discussed.
Co-reporter:Joshua D. Neukom ; Nicholas S. Perch
Journal of the American Chemical Society 2010 Volume 132(Issue 18) pp:6276-6277
Publication Date(Web):April 16, 2010
DOI:10.1021/ja9102259
The synthesis of (dppf)Pd(C6H4-p-F)[N(Ar1)(CH2)3CH═CH2] complexes (3), which are thought to be intermediates in Pd-catalyzed alkene carboamination reactions, is described. These complexes undergo syn-migratory insertion of the alkene into the Pd−N bond to yield observable (dppf)palladium(aryl)(pyrrolidin-2-yl-methyl) complexes 6. Reductive elimination from 6 provides 2-benzylpyrrolidine derivatives 4. The rates of conversion of 3 to 6 (k1) and 6 to 4 (k2) were measured and are within 1 order of magnitude of each other. The syn-migratory insertion stereochemistry was confirmed through a deuterium labeling experiment. These are the first examples of syn-migratory insertions of unactivated alkenes into Pd−N bonds of well-defined complexes.
Co-reporter:Danielle M. Schultz and John P. Wolfe
Organic Letters 2010 Volume 12(Issue 5) pp:1028-1031
Publication Date(Web):February 8, 2010
DOI:10.1021/ol100033s
A new method for the synthesis of tricyclic nitrogen heterocycles from N,2-diallylaniline derivatives is described. These transformations proceed via sequential alkene aminopalladation of an intermediate LnPd(Ar)(NRR′) species followed by alkene carbopalladation of the resulting LnPd(Ar)(R) complex. Both alkene insertion steps occur in preference to C−N or C−C bond-forming reductive elimination. An unusual 1,3-palladium shift occurs when 2-Allyl-N-(2-vinylphenyl)aniline is employed as substrate, which yields a tetracyclic molecule with three contiguous stereocenters.
Co-reporter:Amanda F. Ward and John P. Wolfe
Organic Letters 2010 Volume 12(Issue 6) pp:1268-1271
Publication Date(Web):February 19, 2010
DOI:10.1021/ol1001472
A highly diastereoselective synthesis of substituted tetrahydrofurans bearing stereocenters at C2 and C1′ via Pd-catalyzed carboetherification reactions of acyclic internal alkenes is described. Use of an improved catalyst composed of Pd2(dba)3/S-Phos provides products with up to >20:1 dr. The stereoselective preparation of tetrahydrofurans containing three stereocenters, including a molecule structurally related to simplakidine A, is also reported.
Co-reporter:Georgia S. Lemen and John P. Wolfe
Organic Letters 2010 Volume 12(Issue 10) pp:2322-2325
Publication Date(Web):April 20, 2010
DOI:10.1021/ol1006828
Palladium-catalyzed carboamination reactions between aryl bromides and 4-(but-3-enyl)-substituted oxazolidin-2-ones are described. These transformations afford bicyclic oxazolidin-2-one derivatives that can be converted to trans-2,5-disubstituted pyrrolidines in one step. The starting materials are easily prepared from amino acid precursors, and products that contain up to three stereocenters are generated with >20:1 dr.
Co-reporter:NatalieC. Giampietro ;JohnP. Wolfe
Angewandte Chemie 2010 Volume 122( Issue 16) pp:2984-2986
Publication Date(Web):
DOI:10.1002/ange.201000609
Co-reporter:NatalieC. Giampietro ;JohnP. Wolfe
Angewandte Chemie International Edition 2010 Volume 49( Issue 16) pp:2922-2924
Publication Date(Web):
DOI:10.1002/anie.201000609
Co-reporter:Brandon R. Rosen, Joshua E. Ney and John P. Wolfe
The Journal of Organic Chemistry 2010 Volume 75(Issue 8) pp:2756-2759
Publication Date(Web):March 18, 2010
DOI:10.1021/jo100344k
The development of conditions that allow use of inexpensive aryl chlorides as electrophiles in Pd-catalyzed alkene carboamination and carboetherification reactions is described. A catalyst composed of Pd(OAc)2 and S-Phos minimizes N-arylation of the substrate and prevents formation of mixtures of regioisomeric products. A number of heterocycles, including pyrrolidines, isoxazolidines, tetrahydrofurans, and pyrazolidines, are efficiently generated with this method.
Co-reporter:Natalie C. Giampietro ; Jeff W. Kampf
Journal of the American Chemical Society 2009 Volume 131(Issue 35) pp:12556-12557
Publication Date(Web):August 14, 2009
DOI:10.1021/ja905930s
A new method for the asymmetric synthesis of α-alkyl-α,β-dihydroxy esters that involves tandem Wittig rearrangement/aldol reactions of O-benzyl- or O-allylglycolate esters derived from 2-phenylcyclohexanol is described. This sequence constructs two C−C bonds and two stereocenters, one of which is quaternary, to afford syn diol products with excellent stereocontrol. Cleavage of the chiral auxiliary affords enantiomerically enriched products with up to 95% ee. The application of this method to the preparation of a key intermediate in the synthesis of the antifungal agent alternaric acid is also described.
Co-reporter:Amanda F. Ward and John P. Wolfe
Organic Letters 2009 Volume 11(Issue 10) pp:2209-2212
Publication Date(Web):April 20, 2009
DOI:10.1021/ol900594h
A five step-synthesis of fused bis-tetrahydrofurans and attached bis-tetrahydrofurans from butadiene diepoxide is described. Two sequential Pd-catalyzed carboetherification reactions between protected 1,2-diols and aryl/alkenyl bromides, each of which form both a C−O bond and a C−C bond, are used to generate the heterocyclic rings with >20:1 dr. Installation of different R1 and R2 groups is achieved in a straightforward fashion through use of different aryl or alkenyl bromide coupling partners.
Co-reporter:Josephine S. Nakhla, Danielle M. Schultz, John P. Wolfe
Tetrahedron 2009 65(33) pp: 6549-6570
Publication Date(Web):
DOI:10.1016/j.tet.2009.04.017
Co-reporter:Matthew L. Leathen, Brandon R. Rosen and John P. Wolfe
The Journal of Organic Chemistry 2009 Volume 74(Issue 14) pp:5107-5110
Publication Date(Web):June 1, 2009
DOI:10.1021/jo9007223
A four-step synthesis of cis-3,5-disubstituted morpholines from enantiomerically pure amino alcohols is described. The key step in the synthesis is a Pd-catalyzed carboamination reaction between a substituted ethanolamine derivative and an aryl or alkenyl bromide. The morpholine products are generated as single stereoisomers in moderate to good yield. This strategy also provides access to fused bicyclic morpholines as well as 2,3- and 2,5-disubstituted products.
Co-reporter:John P. Wolfe
European Journal of Organic Chemistry 2007 Volume 2007(Issue 4) pp:571-582
Publication Date(Web):13 NOV 2006
DOI:10.1002/ejoc.200600767
Substituted tetrahydrofuran and pyrrolidine moieties are displayed in a wide range of interesting biologically active molecules. The Pd-catalyzed carboetherification or carboamination of γ-hydroxy and γ-aminoalkenes is a powerful tool for the construction of these heterocycles, as it is convergent and can allow access to a variety of analogs from a single γ-hydroxy- or γ-aminoalkene starting material. This microreview describes the current state of this field. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2007)
Co-reporter:Michael B. Hay;John P. Wolfe
Angewandte Chemie 2007 Volume 119(Issue 34) pp:
Publication Date(Web):26 JUL 2007
DOI:10.1002/ange.200701386
Mehr als eine Alternative: Carboveretherungen von N-Butenylhydroxylaminen mit Bromarenen liefern substituierte Isoxazolidine in guten Ausbeuten und bis d.r. >20:1 (siehe Schema). Diese Umsetzungen bilden eine neue Strategie zum Aufbau von Isoxazolidinen und ermöglichen auch die Synthese von Isoxazolidin-Stereoisomeren, die mit herkömmlichen Methoden nicht herstellbar sind.
Co-reporter:Michael B. Hay;John P. Wolfe
Angewandte Chemie International Edition 2007 Volume 46(Issue 34) pp:
Publication Date(Web):26 JUL 2007
DOI:10.1002/anie.200701386
Pd ploy to isoxazolidines: Carboetherification reactions of N-butenylhydroxylamines with aryl bromides afford substituted isoxazolidines in good yield with up to >20:1 d.r. These transformations provide a new strategy for the construction of isoxazolidines and allow for the synthesis of isoxazolidine stereoisomers that cannot be prepared with existing methods.
Co-reporter:Joshua E. Ney;Michael B. Hay;Qifei Yang;John P. Wolfe
Advanced Synthesis & Catalysis 2005 Volume 347(Issue 11-13) pp:
Publication Date(Web):19 OCT 2005
DOI:10.1002/adsc.200505172
A palladium-catalyzed carboamination reaction of γ-N-arylamino alkenes with vinyl bromides that affords N-aryl-2-allyl pyrrolidines is described. These reactions proceed with high diastereoselectivity for the formation of trans-2,3- and cis-2,5-disubstituted pyrrolidines. Conditions for a tandem N-arylation/carboamination sequence that leads to the formation of an N-aryl-2-allyl pyrrolidine or indoline via the coupling of a primary γ-amino alkene, an aryl bromide, and a vinyl bromide are also reported. The mechanism of the carboamination reactions and the origin of unexpected products that formally derive from rearrangement of the vinyl bromide are discussed.
Co-reporter:Joshua E. Ney
Angewandte Chemie International Edition 2004 Volume 43(Issue 27) pp:
Publication Date(Web):29 JUN 2004
DOI:10.1002/anie.200460060
The formation of a CC and a CN bond in a reaction between γ-(N-arylamino) alkenes and aryl bromides results in the stereoselective synthesis of substituted pyrrolidine derivatives (see scheme). Preliminary studies suggest these reactions proceed by intramolecular alkene insertion into the PdN bond of intermediate [Pd(Ar)(amido)] complexes. dba=dibenzylideneacetone, dppb=1,3-bis(diphenylphosphanyl)butane.
Co-reporter:Joshua E. Ney
Angewandte Chemie 2004 Volume 116(Issue 27) pp:
Publication Date(Web):29 JUN 2004
DOI:10.1002/ange.200460060
Die Bildung einer C-C- und einer C-N-Bindung bei einer Reaktion zwischen γ-N-Arylaminoalkenen und Arylbromiden führt zur stereoselektiven Synthese substituierter Pyrrolidinderivate (siehe Schema). Erste Studien legen nahe, dass diese Reaktionen über eine intramolekulare Alkeninsertion in die Pd-N-Bindung von [Pd(Ar)(amido)]-Intermediaten verlaufen. dba=Dibenzylidenaceton, dppb=1,3-Bis(diphenylphosphanyl)butan.
Co-reporter:B. A. Hopkins and J. P. Wolfe
Chemical Science (2010-Present) 2014 - vol. 5(Issue 12) pp:NaN4844-4844
Publication Date(Web):2014/08/27
DOI:10.1039/C4SC01327A
A catalyst composed of Pd2(dba)3 and (S)-Siphos-PE provides excellent results in Pd-catalyzed alkene carboamination reactions between aniline derivatives bearing pendant alkenes and aryl or alkenyl halides. These transformations generate tetrahydroquinolines and tetrahydroquinoxalines that contain quaternary carbon stereocenters with high levels of asymmetric induction. In addition this catalyst also effects the asymmetric synthesis of tetrahydroisoquinolines via related transformations of 2-allylbenzylamines. In contrast to most other approaches to the asymmetric synthesis of these compounds, which frequently involve functional group interconversion or a single C–C or C–N bond-forming event, the carboamination reactions generate both a C–N bond, a C–C bond, and a stereocenter.
Co-reporter:Amanda F. Ward, Yan Xu and John P. Wolfe
Chemical Communications 2012 - vol. 48(Issue 4) pp:NaN611-611
Publication Date(Web):2011/11/24
DOI:10.1039/C1CC15880E
A new method for the construction of 2-substituted and 2,2-disubstituted chromans viaPd-catalyzed carboetherification reactions between aryl/alkenyl halides and 2-(but-3-en-1-yl)phenols is described. These reactions effect formation of a C–O bond and a C–C bond to afford the chroman products in good yield, and also provide stereoselective access to tricyclic chroman derivatives.
Co-reporter:Johnathon T. Hutt and John P. Wolfe
Inorganic Chemistry Frontiers 2016 - vol. 3(Issue 10) pp:NaN1318-1318
Publication Date(Web):2016/08/22
DOI:10.1039/C6QO00215C
A new Pd-catalyzed alkene carboalkoxylation strategy for the preparation of 2,3-dihydrobenzofurans is described. This method effects the coupling of readily available 2-allylphenol derivatives with aryl triflates to generate a wide range of functionalized 2,3-dihydrobenzofurans in good yields and diastereoselectivities (up to >20:1). Use of newly developed reaction conditions that promote anti-heteropalladation of the alkene is essential in order to generate products in high yield.