Co-reporter:Jihye Lee, Yijiang Shi, Mario Vega, Yonghui Yang, Joseph Gera, Michael E. Jung, Alan Lichtenstein
Bioorganic & Medicinal Chemistry Letters 2017 Volume 27, Issue 20(Issue 20) pp:
Publication Date(Web):15 October 2017
DOI:10.1016/j.bmcl.2017.09.002
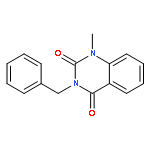
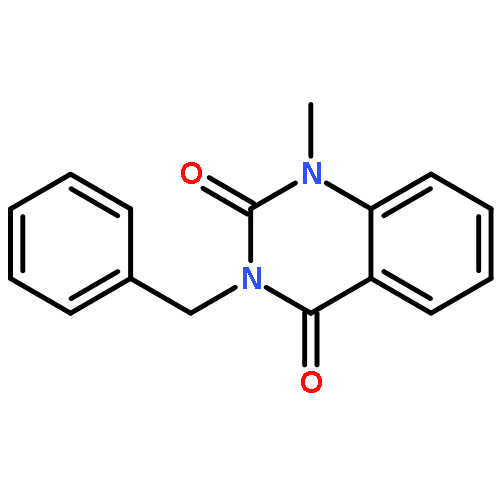
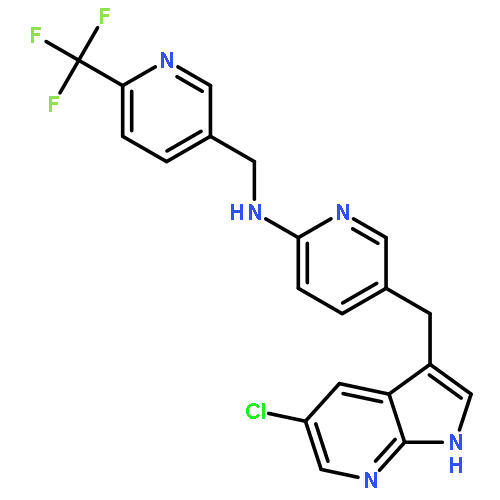
DEPTOR is a 48 kDa protein that binds to mTOR and inhibits this kinase within mTORC1 and mTORC2 complexes. Over-expression of DEPTOR specifically occurs in the multiple myeloma (MM) tumor model and DEPTOR knockdown is cytotoxic to MM cells, suggesting it is a potential therapeutic target. Since mTORC1 paralysis protects MM cells against DEPTOR knockdown, it indicates that the protein–protein interaction between DEPTOR and mTOR is key to MM viability vs death. In a previous study, we used a yeast two-hybrid screen of a small inhibitor library to identify a compound that inhibited DEPTOR/mTOR binding in yeast. This therapeutic (compound B) also prevented DEPTOR/mTOR binding in MM cells and was selectively cytotoxic to MM cells. We now present a structure–activity relationship (SAR) study around this compound as a follow-up report of this previous work. This study has led to the discovery of five new leads – namely compounds 3g, 3k, 4d, 4e and 4g – all of which have anti-myeloma cytotoxic properties superior to compound B. Due to their targeting of DEPTOR, these compounds activate mTORC1 and selectively induce MM cell apoptosis and cell cycle arrest.Download high-res image (59KB)Download full-size image
Co-reporter:Michael E. Jung, Courtney A. Roberts, Felix Perez, Hung V. Pham, Lufeng Zou, and K. N. Houk
Organic Letters 2016 Volume 18(Issue 1) pp:32-35
Publication Date(Web):December 15, 2015
DOI:10.1021/acs.orglett.5b03112
The rearrangements of 4-substituted bicyclo[2.2.2]oct-5-en-2-yl radicals, generated from the corresponding Diels–Alder adducts with phenylseleno acrylates by radical-induced reductive deselenocarbonylations, give the 2-substituted bicyclo[3.2.1]oct-6-en-2-yl radicals with some substituents, e.g., alkoxy and phenyl, but not for silyloxymethyl or benzyl substituents. Theoretical calculations with DFT give the thermodynamics of these reactions and the origins of these processes.
Co-reporter:Michael E. Jung, Daniel L. Sun, Timothy A. Dwight, Peiyuan Yu, Wei Li, and K. N. Houk
Organic Letters 2016 Volume 18(Issue 19) pp:5138-5141
Publication Date(Web):September 12, 2016
DOI:10.1021/acs.orglett.6b02588
The efficient synthesis of trans-2-ethenylcyclopropyl aryl ketones via an intramolecular SN2-like displacement of an allylic ester is reported. A novel 1,5-acyl shift process is also observed that contributes to the product mixture. Theoretical calculations provide a rationale for the observed product ratio.
Co-reporter:Michael E. Jung, Brian T. Chamberlain, Pierre Koch, and Kayvan R. Niazi
Organic Letters 2015 Volume 17(Issue 14) pp:3608-3611
Publication Date(Web):July 6, 2015
DOI:10.1021/acs.orglett.5b01712
An analogue 2 of Brasilicardin A, 1 (BraA), a potent immunosuppressive and cytotoxic agent, was synthesized in which the natural tricyclic skeleton was replaced with a synthetically more accessible substituted tetrahydronaphthalene core. BraA, this analogue (BraL), and cyclosporine A were tested for their ability to inhibit the proliferation of human T cells upon CD3/CD28 activation. Although BraL did not impact T cell activation over the dose range tested, this study shows the inhibitory activity of BraA on human T cells for the first time.
Co-reporter:Nikolai M. Evdokimov; Peter M. Clark; Graciela Flores; Timothy Chai; Kym F. Faull; Michael E. Phelps; Owen N. Witte∞
Journal of Medicinal Chemistry 2015 Volume 58(Issue 14) pp:5538-5547
Publication Date(Web):June 23, 2015
DOI:10.1021/acs.jmedchem.5b00569
Life-threatening acute liver failure can be triggered by a variety of factors, including common drugs such as acetaminophen. Positron emission tomography (PET) is rarely used to monitor liver function, in part because of a lack of specific imaging agents for liver function. Here we report a new PET probe, 2-deoxy-2-[18F]fluororibose ([18F]-2-DFR), for use in imaging liver function. [18F]-2-DFR was synthesized and validated as a competitive substrate for the ribose salvage pathway. [18F]-2-DFR was prepared through an efficient late stage radiofluorination. The desired selectivity of fluorination was achieved using an unorthodox protecting group on the precursor, which could withstand harsh SN2 reaction conditions with no side reactions. [18F]-2-DFR accumulated preferentially in the liver and was metabolized by the same enzymes as ribose. [18F]-2-DFR could distinguish between healthy liver and liver damaged by acetaminophen. [18F]-2-DFR is expected to be a useful PET probe for imaging and quantifying liver functions in vivo, with likely significant clinical utility.
Co-reporter:Michael E. Jung, Daniel L. Sun
Tetrahedron Letters 2015 Volume 56(Issue 23) pp:3082-3085
Publication Date(Web):3 June 2015
DOI:10.1016/j.tetlet.2014.11.103
The intermediate epoxy alcohols prepared via a Payne rearrangement can be trapped with arylselenide anions, giving mixtures of ring-opened products. The 1-arylseleno-2,3-diols are generally favored over the 3-arylseleno-1,2-diols in this process although the reaction of trisubstituted epoxyalcohols, for example, 17, differs from those of disubstituted epoxyalcohols, for example, 21.
Co-reporter:Michael E. Jung, Gloria S. Lee, Hung V. Pham, and K. N. Houk
Organic Letters 2014 Volume 16(Issue 9) pp:2382-2385
Publication Date(Web):April 11, 2014
DOI:10.1021/ol500710v
The exomethylenes of 2,6-disubstituted bicyclo[3.3.1]nonan-9-ones 2 are readily isomerized over a palladium catalyst under an atmosphere of hydrogen to predominantly form the isomer 3 with C2 symmetry with very little formation of the analogous product with Cs symmetry. A hydrogen source is essential to effect the rearrangement.
Co-reporter:Michael E. Jung and Gang Deng
Organic Letters 2014 Volume 16(Issue 8) pp:2142-2145
Publication Date(Web):April 1, 2014
DOI:10.1021/ol500592m
Both alkylarylalkynes and diarylalkynes 1 are converted into the α-diketones 2 in good yield by the use of mercuric salts, e.g., mercuric nitrate hydrate or mercuric triflate, in the presence of water. Other mercuric salts, e.g., sulfate, chloride, acetate, or trifluoroacetate, do not provide the diketone product. A possible mechanism is proposed.
Co-reporter:Michael E. Jung, Brian T. Chamberlain, Chi-Lee C. Ho, Eugene J. Gillespie, and Kenneth A. Bradley
ACS Medicinal Chemistry Letters 2014 Volume 5(Issue 4) pp:363-367
Publication Date(Web):January 21, 2014
DOI:10.1021/ml400486k
EGA, 1, prevents the entry of multiple viruses and bacterial toxins into mammalian cells by inhibiting vesicular trafficking. The cellular target of 1 is unknown, and a structure–activity relationship study was conducted in order to develop a strategy for target identification. A compound with midnanomolar potency was identified (2), and three photoaffinity labels were synthesized (3–5). For this series, the expected photochemistry of the phenyl azide moiety is a more important factor than the IC50 of the photoprobe in obtaining a successful photolabeling event. While 3 was the most effective reversible inhibitor of the series, it provided no protection to cells against anthrax lethal toxin (LT) following UV irradiation. Conversely, 5, which possessed weak bioactivity in the standard assay, conferred robust irreversible protection vs LT to cells upon UV photolysis.Keywords: anthrax lethal toxin; aryl azide; endosomal trafficking; Photoaffinity labeling; semicarbazone;
Co-reporter:Michael E. Jung and Gloria S. Lee
The Journal of Organic Chemistry 2014 Volume 79(Issue 21) pp:10547-10552
Publication Date(Web):October 2, 2014
DOI:10.1021/jo501368d
Trifluoromethanesulfonic acid and other electrophiles promote formation of the adamantanone core from the readily accessible 1,5-dimethyl-3,7-dimethylenebicyclo[3.3.1]nonan-9-one 2. Because adamantyl cation 3 can be trapped by a range of nucleophiles, including aromatic and heteroaromatic rings, alcohol, nitriles, and halides, access to a wide variety of functionality at the newly formed tertiary position is provided.
Co-reporter:Michael E. Jung;Timothy A. Dwight;Frederic Vigant;Michael E. Østergaard;Eric E. Swayze;Punit P. Seth
Angewandte Chemie International Edition 2014 Volume 53( Issue 37) pp:9893-9897
Publication Date(Web):
DOI:10.1002/anie.201405283
Abstract
The efficient synthesis, antiviral activity, and duplex-stabilizing properties of both isomers of the 2′-fluoro analogue of Northern methanocarbathymidine (N-MCT), 2 and 3, are reported. We show that 2′-F incorporation on the N-MCT scaffold has a strong stabilizing effect on duplex thermal stability.
Co-reporter:Michael E. Jung;Timothy A. Dwight;Frederic Vigant;Michael E. Østergaard;Eric E. Swayze;Punit P. Seth
Angewandte Chemie 2014 Volume 126( Issue 37) pp:10051-10055
Publication Date(Web):
DOI:10.1002/ange.201405283
Abstract
The efficient synthesis, antiviral activity, and duplex-stabilizing properties of both isomers of the 2′-fluoro analogue of Northern methanocarbathymidine (N-MCT), 2 and 3, are reported. We show that 2′-F incorporation on the N-MCT scaffold has a strong stabilizing effect on duplex thermal stability.
Co-reporter:Wei Zhong ; James R. Springstead ; Ramea Al-Mubarak ; Sangderk Lee ; Rongsong Li ; Benjamin Emert ; Judith A. Berliner
Journal of Medicinal Chemistry 2013 Volume 56(Issue 21) pp:8521-8532
Publication Date(Web):October 13, 2013
DOI:10.1021/jm400959q
The goal of these studies was to determine the effect of 5,6-epoxyisoprostane, EI, on human aortic endothelial cells (HAEC). EI can form as a phospholipase product of 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphocholine, PEIPC, a proinflammatory molecule that accumulates in sites of inflammation where phospholipases are also increased. To determine the effect of EI on HAEC, we synthesized several stereoisomers of EI using a convergent approach from the individual optically pure building blocks, the epoxyaldehydes 5 and 6 and the bromoenones 14 and 16. The desired stereoisomer of EI can be prepared from these materials in only six operations, and thus, large amounts of the product can be obtained. The trans/trans isomers had the most potent activity, suggesting specificity in the interaction of EI with the cell surface. EI has potent anti-inflammatory effects in HAEC. EI strongly inhibits the production of MCP-1, a major monocyte chemotactic factor, and either decreases or minimally increases the levels of 10 proinflammatory molecules increased by PEIPC. EI also strongly down-regulates the inflammatory effects of IL-1β, a major inflammatory cytokine. Thus EI, a hydrolytic product of PEIPC, has potent anti-inflammatory function.
Co-reporter: Michael E. Jung;Felix Perez;Dr. Collin F. Regan;Dr. Sung Wook Yi ;Dr. Quentin Perron
Angewandte Chemie 2013 Volume 125( Issue 7) pp:2114-2116
Publication Date(Web):
DOI:10.1002/ange.201208294
Co-reporter: Michael E. Jung;Felix Perez;Dr. Collin F. Regan;Dr. Sung Wook Yi ;Dr. Quentin Perron
Angewandte Chemie International Edition 2013 Volume 52( Issue 7) pp:2060-2062
Publication Date(Web):
DOI:10.1002/anie.201208294
Co-reporter:Michael E. Jung and Mikhail Guzaev
The Journal of Organic Chemistry 2013 Volume 78(Issue 15) pp:7518-7526
Publication Date(Web):July 8, 2013
DOI:10.1021/jo400909t
Studies toward the enantiospecific total synthesis of rhodexin A via a very hindered inverse electron demand Diels–Alder reaction are described. The C8-diastereomer of the fully elaborated tetracyclic core of rhodexin A, 23, was prepared in good yield and excellent selectivity using as the key step the stepwise Diels–Alder reaction of the very hindered dienone 3 and the silyl enol ether 4 catalyzed by the very strong Lewis acid, dimethylaluminum triflimide.
Co-reporter:Michael E. Jung and Mikhail Guzaev
Organic Letters 2012 Volume 14(Issue 20) pp:5169-5171
Publication Date(Web):October 3, 2012
DOI:10.1021/ol302172y
Two catalysts, Me2AlNTf2 and MeAl(NTf2)2, derived from the mixing of trimethylaluminum with triflimide, proved to be highly effective catalysts in hindered Diels–Alder reactions, generating the desired Diels–Alder cycloadducts from both hindered 2-silyloxydienes and hindered dienophiles. Thus reaction of 1 with 2 afforded the hindered cycloadduct 4 in excellent yield in 0.5–1.5 h at −40 °C.
Co-reporter:Michael E. Jung and Jonah J. Chang
Organic Letters 2012 Volume 14(Issue 18) pp:4898-4901
Publication Date(Web):September 6, 2012
DOI:10.1021/ol302234a
The proposed structure of the fungal metabolite, mycosporulone 1, was prepared starting from the cyclohexenone ester 11 and the d-(R)-glyceraldehyde acetonide 12. The spectroscopic data for both 1 and its C2 epimer 1a did not match those reported for the natural product. A revised structure 29 for mycosporulone is proposed.
Co-reporter:Michael E. Jung, Sung Wook Yi
Tetrahedron Letters 2012 Volume 53(Issue 32) pp:4216-4220
Publication Date(Web):8 August 2012
DOI:10.1016/j.tetlet.2012.05.153
Chelation-controlled addition of organocuprates to N-carbamoyl aminoaldehydes, prepared from functionalized amino acids, generated predominately the threo-β-amino alcohol derivatives through chelation with the carbamoyl moiety. The carbamate group is a stronger chelating group than other potentially good chelators, for example ethers, esters, thioethers, and gives good diastereoselectivity with cuprates. Thus addition of lithium divinylcuprate to the aldehyde generated from the serine derivative 25 in the presence of extra copper for chelation afforded the threo compound 26 in 83% yield. Cross-metathesis and cleavage of the protecting groups furnished l-threo sphingosine 21. In addition the lyso-sphingolipid protein kinase C inhibitor, safingol, 22, was prepared from commercially available O-benzyl N-BOC serine 28 in six steps and 56% overall yield by this method.
Co-reporter:Michael E. Jung and Gang Deng
The Journal of Organic Chemistry 2012 Volume 77(Issue 23) pp:11002-11005
Publication Date(Web):November 19, 2012
DOI:10.1021/jo302308q
Oxidative cleavage of cycloalkene-1-carboxylates, made from the corresponding carboxylic acids, and subsequent oxidation of the resulting ketoaldehyde afforded the important 1-monoesters of 2-ketoalkanedioic acids. Thus ozonolysis of octyl cyclobutene-1-carboxylate followed by sodium chlorite oxidation afforded the 1-monooctyl 2-ketoglutarate. This is a cell-permeable prodrug form of α-ketoglutarate, an important intermediate in the tricarboxylic acid (TCA, Krebs) cycle and a promising therapeutic agent in its own right.
Co-reporter:Hao Wang, K. N. Houk, Damian A. Allen, and Michael E. Jung
Organic Letters 2011 Volume 13(Issue 12) pp:3238-3241
Publication Date(Web):May 13, 2011
DOI:10.1021/ol2011488
The non-aldol aldol reaction of the isomeric epoxy silyl ethers is controlled by the conformation of the transition states leading to an internal hydride shift. One isomer rearranges to the β-silyloxy ketone whereas the other isomer gives a β-elimination product. Theoretical calculations show that the substrates with substituents that favor the formation of the chairlike transition state rearrange normally while those that do not undergo elimination instead.
Co-reporter:Michael E. Jung, Timothy A. Dong, Xiaolu Cai
Tetrahedron Letters 2011 Volume 52(Issue 20) pp:2533-2535
Publication Date(Web):18 May 2011
DOI:10.1016/j.tetlet.2011.02.111
The 7-nitrobenz-2,1,3-oxadiazole (NBD) unit is a highly useful fluorescent tag with wide application in biology. Installation of the NBD group typically proceeds via the SNAr reaction between an amine and an NBD halide. Herein, we demonstrate that NBD-F 1 results in significantly higher yields than NBD-Cl 2, and that triethylamine in dimethylformamide at 23 °C overnight is a broadly applicable set of conditions for this reaction. In particular, the highly useful fluorescent carbohydrate 2-NBD-glucosamine (2-NBDG, 3) can now be prepared in 75% yield with NBD-F as compared to 12% with NBD-Cl.NBD-F 1 gives much higher yields with amines than does NBD-Cl 2 giving the NBD amines, for example, 2-NBD-glucosamine, 3.
Co-reporter:Michael E. Jung, Pierre Koch
Tetrahedron Letters 2011 Volume 52(Issue 46) pp:6051-6054
Publication Date(Web):16 November 2011
DOI:10.1016/j.tetlet.2011.08.102
An efficient method for the cleavage of the p-methoxybenzyl protecting group of several alcohols in the presence of 0.5 equiv of trifluoromethanesulfonic acid and 1,3-dimethoxybenzene in dichloromethane at room temperature is described.Triflic acid and 1,3-dimethoxybenzene in dichloromethane readily cleaves p-methoxybenzyl (PMB) ethers at room temperature, for example, 13 affords 14 in 98% yield.
Co-reporter:Michael E. Jung, Hiufung V. Chu
Tetrahedron Letters 2011 Volume 52(Issue 35) pp:4512-4514
Publication Date(Web):31 August 2011
DOI:10.1016/j.tetlet.2011.06.114
A concise synthesis of novel cardiac glycoside analogues of rhodexin A, 14 and 24, having the BCD tricyclic system is described. The key constructive step is an inverse-electron demand Diels–Alder reaction of the silyl enol ether 4 and the 2-acetyldiene, 7 and 15.A concise synthesis of the two BCD tricyclic analogues 14 and 24 of the cardiac glycoside rhodexin A is described.
Co-reporter:Michael E. Jung, Jin-Mo Ku, Liutao Du, Hailiang Hu, Richard A. Gatti
Bioorganic & Medicinal Chemistry Letters 2011 21(19) pp: 5842-5848
Publication Date(Web):
DOI:10.1016/j.bmcl.2011.07.107
Co-reporter:Michael E. Jung, Dongwon Yoo
Tetrahedron 2011 67(52) pp: 10281-10286
Publication Date(Web):
DOI:10.1016/j.tet.2011.10.024
Co-reporter:Michael E. Jung and Jonah J. Chang
Organic Letters 2010 Volume 12(Issue 13) pp:2962-2965
Publication Date(Web):June 1, 2010
DOI:10.1021/ol1009762
The diastereospecific attack of the silyl enol ether on the activated cyclopropyl diester 27 generated the hydrindanone 28 with complete stereocontrol. Thermal decarbomethoxylation of 28 gave the monoester 29, a key intermediate in Heathcock’s synthesis, thereby completing a formal total synthesis of (+)-fawcettimine 1. The analogous cyclization of 33, the diastereomer of 27, afforded the diastereomeric diester 34, thereby demonstrating that the cyclization process is diastereospecific.
Co-reporter:Michael E. Jung, Manon Chaumontet and Ramin Salehi-Rad
Organic Letters 2010 Volume 12(Issue 12) pp:2872-2875
Publication Date(Web):May 25, 2010
DOI:10.1021/ol100985n
A non-aldol aldol−cuprate opening generates the polypropionate 11 from the epoxy ether 14 in eight steps as a single diastereomer. A highly stereoselective aldol reaction of 8 with 9 gives the aldol product 7 in high yield and excellent diastereoselectivity, due to double stereodifferentiation. This compound was used for an efficient synthesis of the natural product auripyrone B 2 in only 20 steps and 8% overall yield from 14 using a late-stage spiroketalization onto a stable hemiketal as the final key step.
Co-reporter:Michael E. Jung ; Samedy Ouk ; Dongwon Yoo ; Charles L. Sawyers ; Charlie Chen ; Chris Tran ;John Wongvipat
Journal of Medicinal Chemistry 2010 Volume 53(Issue 7) pp:2779-2796
Publication Date(Web):March 10, 2010
DOI:10.1021/jm901488g
A structure−activity relationship study was carried out on a series of thiohydantoins and their analogues 14 which led to the discovery of 92 (MDV3100) as the clinical candidate for the treatment of hormone refractory prostate cancer.
Co-reporter:Michael E. Jung, Ramin Salehi-Rad
Tetrahedron Letters 2010 Volume 51(Issue 38) pp:4931-4933
Publication Date(Web):22 September 2010
DOI:10.1016/j.tetlet.2010.07.034
An approach to the synthesis of the cytotoxic natural product auripyrone A 1 via the cyclization of an alcohol onto a γ-pyrone in 3 is described. The bis(pyrone) alcohol 3 was prepared efficiently from the advanced aldolate 4 via silyl ether cleavage, oxidation, pyrone formation, and PMB ether removal. Instead of providing auripyrone A 1, the attempted cyclization of 3 gave the product of 1,5-acyl migration 8. Model studies show this to be a general process; therefore, cyclization of an alcohol on such a hindered γ-pyrone under normal conditions is very difficult.Treatment of the bis(pyrone) alcohol 3 with an acid did not give the desired cytotoxic product, auripyrone A, 1, but rather an acyl transfer to give 8. Model studies show this to be a general process and therefore such a cyclization is very difficult.
Co-reporter:Michael E. Jung, Ting-Hu Zhang, Rebecca M. Lui, Osvaldo Gutierrez, and K. N. Houk
The Journal of Organic Chemistry 2010 Volume 75(Issue 20) pp:6933-6940
Publication Date(Web):September 15, 2010
DOI:10.1021/jo101533h
While thermolysis of the macrobicyclic triene lactone 12 did not produce the expected bicyclic transannular Diels−Alder (BTADA) product 13, heating the corresponding ether 18 to 110 °C for 4 h afforded a quantitative yield of the desired cycloadduct 19, which could be easily reduced to the perhydrophenanthrene, an ABC ring analogue of fusidic acid 1. Theoretical calculations with hybrid density functional theory (B3LYP/6-31G(d)) help rationalize why the lactone does not cyclize whereas the ether does.
Co-reporter:Michael E. Jung, Jesus Cordova and Masayuki Murakami
Organic Letters 2009 Volume 11(Issue 17) pp:3882-3885
Publication Date(Web):August 5, 2009
DOI:10.1021/ol901455q
The total synthesis of the diterpene kellermanoldione 1 is reported. Stepwise [4 + 2] cycloaddition of the ketal diene 8 and the allenoate 3 afforded the exo adduct 10x as the major product. It was converted into 1 via six steps, among them a key nonconjugative hydrolysis of a γ-methylene silyl enol ether.
Co-reporter:Michael E. Jung and Felix Perez
Organic Letters 2009 Volume 11(Issue 10) pp:2165-2167
Publication Date(Web):April 16, 2009
DOI:10.1021/ol900416x
Mukaiyama Michael addition of silyl enol ethers 13 to the 1,2-quinone-4-carboxylate 6 (formed in situ by oxidation of the catechol ester 8) afforded the 2-subsituted 7-hydroxybenzofuran-4-carboxylates 14 in fair to good yields. Alkyl and aryl systems work well, but highly electron-rich silyl enol ethers could not be used because of competing oxidation.
Co-reporter:Chris Tran;Samedy Ouk;Nicola J. Clegg;Yu Chen;Philip A. Watson;Vivek Arora;Andrew Kwon;John Wongvipat;Dongwon Yoo;Peter M. Smith-Jones;Teresa Wasielewska;Derek Welsbie;Charlie Degui Chen;Celestia S. Higano;Tomasz M. Beer;David T. Hung;Howard I. Scher;Charles L. Sawyers
Science 2009 Volume 324(Issue 5928) pp:
Publication Date(Web):
DOI:10.1126/science.1168175
A Second Act for Antiandrogens
Men with advanced prostate cancer are often treated with antiandrogens; drugs that inhibit the activity of male hormones, such as testosterone, that help drive tumor growth. Many of these drugs act by functionally disrupting the androgen receptor (AR), a transcriptional regulator of cell proliferation, but tumors eventually become resistant to the drugs by expressing higher levels of the AR. Tran et al. (p. 787, published online 9 April) have developed a “second-generation” antiandrogen, a thiohydantoin called MDV3100, which binds the AR with high affinity. MDV3100 retains its anticancer activity in cell culture and in mouse models even when AR levels are elevated. The drug appears to act both by inhibiting translocation of the AR into the nucleus and by reducing its transcriptional activity. MDV3100 is being tested in patients with advanced prostate cancer, the first group of which have shown a decline in blood levels of a marker of cancer growth, prostate-specific antigen.
Co-reporter:MichaelE. Jung ;Ramin Salehi-Rad
Angewandte Chemie 2009 Volume 121( Issue 46) pp:8922-8925
Publication Date(Web):
DOI:10.1002/ange.200904607
Co-reporter:MichaelE. Jung ;Ramin Salehi-Rad
Angewandte Chemie International Edition 2009 Volume 48( Issue 46) pp:8766-8769
Publication Date(Web):
DOI:10.1002/anie.200904607
Co-reporter:Nuttee Suree, Sung Wook Yi, William Thieu, Melanie Marohn, Robert Damoiseaux, Albert Chan, Michael E. Jung, Robert T. Clubb
Bioorganic & Medicinal Chemistry 2009 17(20) pp: 7174-7185
Publication Date(Web):
DOI:10.1016/j.bmc.2009.08.067
Co-reporter:Michael E. Jung, Jeremy J. Clemens, Nuttee Suree, Chu Kong Liew, Rosemarie Pilpa, Dean O. Campbell, Robert T. Clubb
Bioorganic & Medicinal Chemistry Letters 2005 Volume 15(Issue 22) pp:5076-5079
Publication Date(Web):15 November 2005
DOI:10.1016/j.bmcl.2005.07.073
l-Threonine 2 was converted in seven steps into the protected aminomercaptoalcohol 8, a threonine mimic. This compound 8 was coupled to various oligopeptides to produce two different tetrapeptide analogues, for example, 11 and 17, which were shown to inhibit the Sortase enzymes (SrtA and SrtB) via covalent attachment of the thiol groups of 11 and 17 to the catalytically active cysteine residue of the Sortase enzymes.
Co-reporter:Michael E. Jung, Annika Kers, Ganesamoorthy Subbanagounder and Judith A. Berliner
Chemical Communications 2003 (Issue 2) pp:196-197
Publication Date(Web):17 Dec 2002
DOI:10.1039/B209892J
We report studies toward the total synthesis of an epoxy isoprostane, namely the preparation of compound 9 which is an analogue of the elimination product 7 of the naturally occurring epoxy isoprostane 4 by a straightforward route using a three-component coupling, and have shown by several spectroscopic criteria that it closely resembles the natural material.
Co-reporter:Michael E. Jung ;Pablo Davidov Dr.
Angewandte Chemie International Edition 2002 Volume 41(Issue 21) pp:
Publication Date(Web):31 OCT 2002
DOI:10.1002/1521-3773(20021104)41:21<4125::AID-ANIE4125>3.0.CO;2-E
The right mix! A 10:1 mixture of AlBr3 and AlMe3 promotes the Diels–Alder reaction of hindered dienophiles. For example, the Diels–Alder reaction of an acetyldiene with a tert-butyldimethylsilyl (TBS) enol ether gave mainly the exo cycloadduct in 77 % yield [Eq. (1); X=COMe, Y=OTBS]. Oxidation, hydrolysis, reduction, and desilylation of this cycloadduct afforded a BCD ring analogue of ouabain.
Co-reporter:Michael E. Jung ;Pablo Davidov Dr.
Angewandte Chemie 2002 Volume 114(Issue 21) pp:
Publication Date(Web):4 NOV 2002
DOI:10.1002/1521-3757(20021104)114:21<4299::AID-ANGE4299>3.0.CO;2-N
Auf die richtige Mischung kommt es an! AlBr3 und AlMe3 im Verhältnis 10:1 vermitteln die Diels-Alder-Reaktion sterisch gehinderter Dienophile. So ergab die Diels-Alder-Reaktion eines Acetyldiens mit einem tert-Butyldimethylsilyl(TBS)-Enolether vorwiegend das exo-Cycloaddukt in 77 % Ausbeute [Gl. (1); X=COMe, Y=OTBS]. Oxidation, Hydrolyse, Reduktion und Desilylierung dieses Cycloaddukts lieferten eine Verbindung, die der BCD-Ring-Substruktur von Ouabain entspricht.





![2,5-Pyrrolidinedione, 1-[(2,4-dimethoxyphenyl)methyl]-](http://img.cochemist.com/ccimg/684300/684287-65-8.png)
![2,5-Pyrrolidinedione, 1-[(2,4-dimethoxyphenyl)methyl]-](http://img.cochemist.com/ccimg/684300/684287-65-8_b.png)


![1H-Pyrrole-2,5-dione, 3,4-dichloro-1-[(2,4-dimethoxyphenyl)methyl]-](http://img.cochemist.com/ccimg/342500/342416-30-2.png)
![1H-Pyrrole-2,5-dione, 3,4-dichloro-1-[(2,4-dimethoxyphenyl)methyl]-](http://img.cochemist.com/ccimg/342500/342416-30-2_b.png)