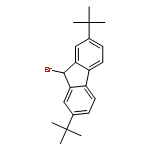
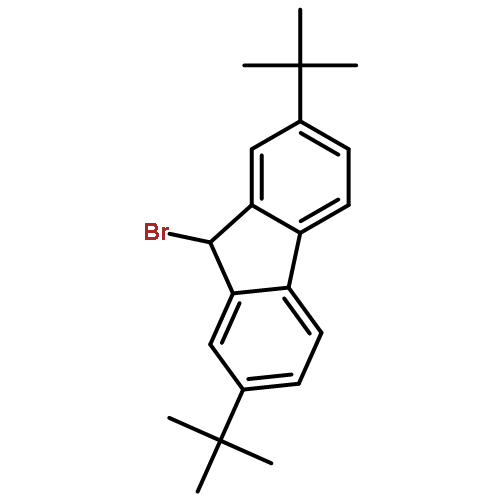
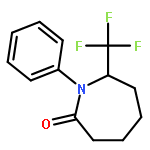
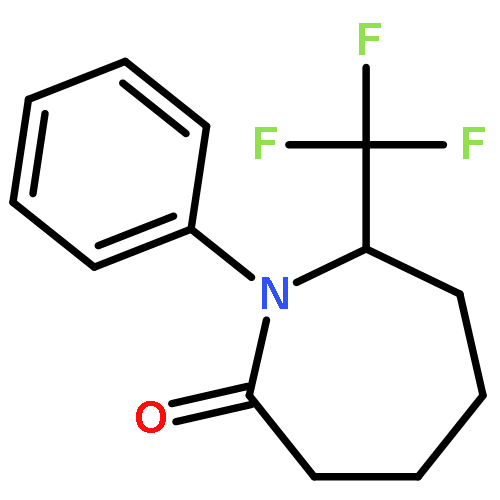
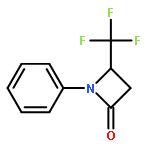
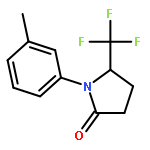
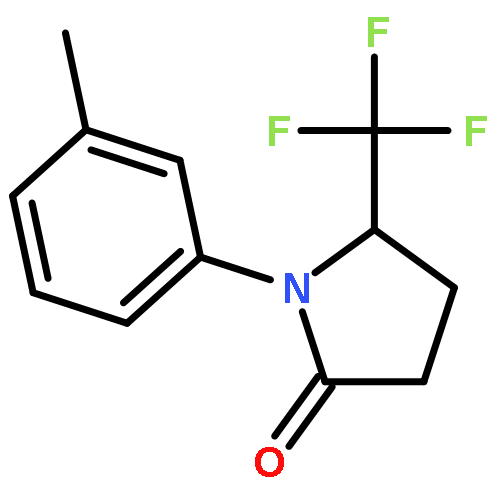
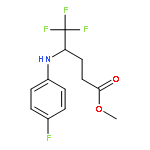
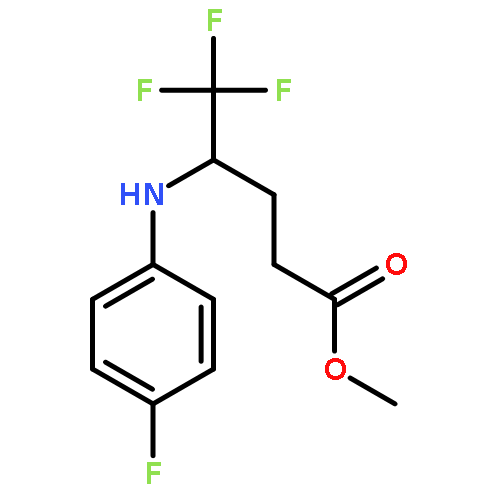
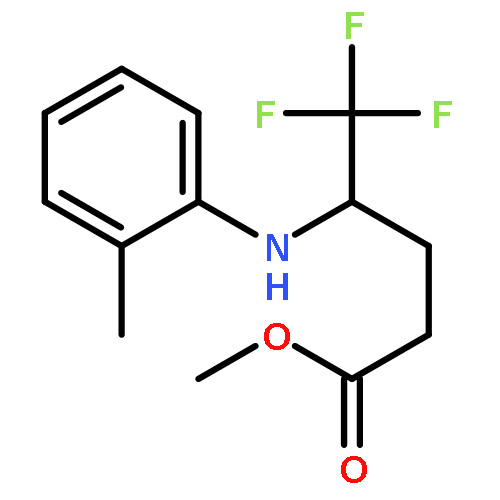
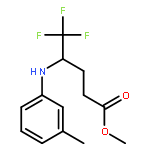
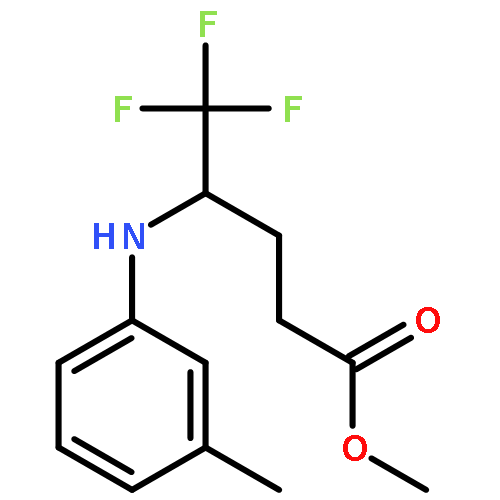
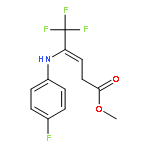
Co-reporter:Wen Wan, Guobin Ma, Jialiang Li, Yunrong Chen, Qingyang Hu, Minjie Li, Haizhen Jiang, Hongmei Deng and Jian Hao
Chemical Communications 2016 vol. 52(Issue 8) pp:1598-1601
Publication Date(Web):30 Nov 2015
DOI:10.1039/C5CC09179A
A mild, versatile and efficient method for the silver(I)-catalyzed oxidative decarboxylative gem-difluoromethylenation has been developed. The radical cascade reaction comprises the addition of an oxidatively generated difluoromethylene radical to the isonitrile functionality and subsequent homolytic aromatic substitution. It provides a novel and efficient access to the C–CF2 bond formation.
Co-reporter:Guobin Ma, Wen Wan, Qingyang Hu, Haizhen Jiang, Jing Wang, Shizheng Zhu and Jian Hao
Chemical Communications 2014 vol. 50(Issue 56) pp:7527-7530
Publication Date(Web):03 Jun 2014
DOI:10.1039/C4CC03321C
A copper-mediated gem-difluoromethylenation of aryl, heteroaryl and vinyl boronic acids with bromodifluoromethylated oxazole or thiazole derivatives has been developed. This novel reaction showed an excellent functional group tolerance and wide substrate scope, providing facile access to practical application in drug discovery and development.
Co-reporter:Guobin Ma, Wen Wan, Jialiang Li, Qingyang Hu, Haizhen Jiang, Shizheng Zhu, Jing Wang and Jian Hao
Chemical Communications 2014 vol. 50(Issue 68) pp:9749-9752
Publication Date(Web):08 Jul 2014
DOI:10.1039/C4CC04591B
A mild, versatile and efficient method for the regioselective hydrodifluoromethylation of unactivated alkenes has been developed. This Ag-mediated Csp3–CF2 bond forming reaction provides easy access to a variety of vicinal α-difluoroacetate-containing alkanes.
Co-reporter:Yunpeng Fan, Wen Wan, Guobin Ma, Wei Gao, Haizhen Jiang, Shizheng Zhu and Jian Hao
Chemical Communications 2014 vol. 50(Issue 43) pp:5733-5736
Publication Date(Web):14 Apr 2014
DOI:10.1039/C4CC01481B
Cu(II)-catalyzed aromatic C–H azidation with azido-benziodoxolone under mild conditions has been described. The primary amine exhibits an excellent ortho-directing effect, providing ortho-azidated anilines as the sole products.
Co-reporter:Wen Wan, Wei Gao, Guobin Ma, Lei Ma, Fan Wang, Jing Wang, Haizhen Jiang, Shizheng Zhu and Jian Hao
RSC Advances 2014 vol. 4(Issue 51) pp:26563-26568
Publication Date(Web):03 Jun 2014
DOI:10.1039/C4RA03362K
A class of chiral bifunctional N-prolyl sulfinamide and its TFA salts were prepared and proven to be effective for catalyzing the aldol reaction under solvent-free conditions. In general, the corresponding aldol adducts were obtained with high to excellent yields, and satisfactory diastereo-selectivities and enantioselectivities. A matching effect between chiral proline and sulfinamide moieties was observed in the catalysts. The enantioswitching of both enantiomers in the asymmetric aldol synthesis is found to be dominated by the prolyl moiety.
Co-reporter:Wen Wan, Heliang Du, Jing Wang, Yuping Le, Haizhen Jiang, Hua Chen, Shizheng Zhu, Jian Hao
Dyes and Pigments 2013 Volume 96(Issue 3) pp:642-652
Publication Date(Web):March 2013
DOI:10.1016/j.dyepig.2012.10.013
A series of twisted fluorenyl anthracenes linked by a rigid sp3-hybridized carbon atom, containing an end-capping aryl substituent at either the C2-position of fluorene or at the C10-position of anthracene, have been synthesized and characterized. These materials exhibited good thermal stabilities and amorphous properties. Theoretical calculations and X-ray diffraction studies revealed that the six blue emitters possess non-coplanar structures to suppress intermolecular interactions. The relationship between the chemical structures with different end-capping groups and the electroluminescent properties has been investigated. Organic light-emitting diodes utilizing 9-(2-phenyl-9H-fluoren-9-yl) anthracene as the emitter exhibited deep-blue emissions CIE x, y (0.166, 0.153) with a current efficiency of 1.47 cd/A, external quantum efficiency of 1.84% at 20 mA/cm2 and a maximum brightness of 3013 cd/m2 at 11 V. It was found that the introduction of the aryl substituents to the C2 position of fluorene could improve the performance of OLEDs based on C9-fluorenyl anthracenes.Graphical abstractHighlights► Six new C9-fluorenyl anthracenes were prepared. ► All have high glass transition temperatures above 100 °C. ► Better performance of OLEDs based on C9-fluorenyl anthracenes with C2-substituents. ► An efficiency (1.47 cd/A) and an external quantum efficiency (1.84%) at 20 mA/cm2.
Co-reporter:Jing Wang, Wen Wan, Haizhen Jiang, Yan Gao, Xueyin Jiang, Huaping Lin, Weiming Zhao and Jian Hao
Organic Letters 2010 Volume 12(Issue 17) pp:3874-3877
Publication Date(Web):August 10, 2010
DOI:10.1021/ol101553a
Syntheses, optical, and electrochemical properties of novel C-9 fluorenyl substituted anthracenes linked by a tetrahedral sp3-hybridized carbon atom are reported for blue light emitting materials. Remarkably, an unoptimized organic light-emitting diode based on 1-fold fluorene-functionalized anthracene 3 exhibits a radiance of 4100 cd/m2 at 12 V and a maximum EL efficiency of 1.36 cd/A with color purity CIE x, y (0.157, 0.082), which is very close to the National Television System Committee standard blue.
Co-reporter:Guobin Ma, Wen Wan, Qingyang Hu, Haizhen Jiang, Jing Wang, Shizheng Zhu and Jian Hao
Chemical Communications 2014 - vol. 50(Issue 56) pp:NaN7530-7530
Publication Date(Web):2014/06/03
DOI:10.1039/C4CC03321C
A copper-mediated gem-difluoromethylenation of aryl, heteroaryl and vinyl boronic acids with bromodifluoromethylated oxazole or thiazole derivatives has been developed. This novel reaction showed an excellent functional group tolerance and wide substrate scope, providing facile access to practical application in drug discovery and development.
Co-reporter:Wen Wan, Guobin Ma, Jialiang Li, Yunrong Chen, Qingyang Hu, Minjie Li, Haizhen Jiang, Hongmei Deng and Jian Hao
Chemical Communications 2016 - vol. 52(Issue 8) pp:NaN1601-1601
Publication Date(Web):2015/11/30
DOI:10.1039/C5CC09179A
A mild, versatile and efficient method for the silver(I)-catalyzed oxidative decarboxylative gem-difluoromethylenation has been developed. The radical cascade reaction comprises the addition of an oxidatively generated difluoromethylene radical to the isonitrile functionality and subsequent homolytic aromatic substitution. It provides a novel and efficient access to the C–CF2 bond formation.
Co-reporter:Guobin Ma, Wen Wan, Jialiang Li, Qingyang Hu, Haizhen Jiang, Shizheng Zhu, Jing Wang and Jian Hao
Chemical Communications 2014 - vol. 50(Issue 68) pp:NaN9752-9752
Publication Date(Web):2014/07/08
DOI:10.1039/C4CC04591B
A mild, versatile and efficient method for the regioselective hydrodifluoromethylation of unactivated alkenes has been developed. This Ag-mediated Csp3–CF2 bond forming reaction provides easy access to a variety of vicinal α-difluoroacetate-containing alkanes.
Co-reporter:Yunpeng Fan, Wen Wan, Guobin Ma, Wei Gao, Haizhen Jiang, Shizheng Zhu and Jian Hao
Chemical Communications 2014 - vol. 50(Issue 43) pp:NaN5736-5736
Publication Date(Web):2014/04/14
DOI:10.1039/C4CC01481B
Cu(II)-catalyzed aromatic C–H azidation with azido-benziodoxolone under mild conditions has been described. The primary amine exhibits an excellent ortho-directing effect, providing ortho-azidated anilines as the sole products.