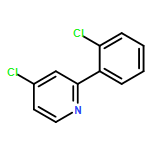
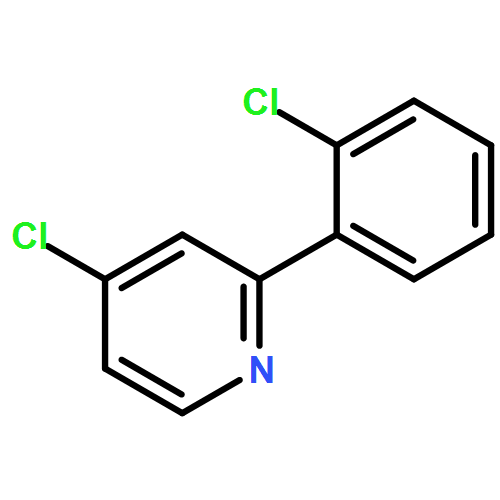
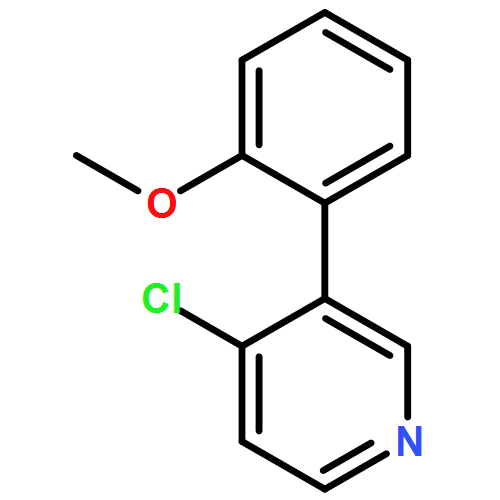
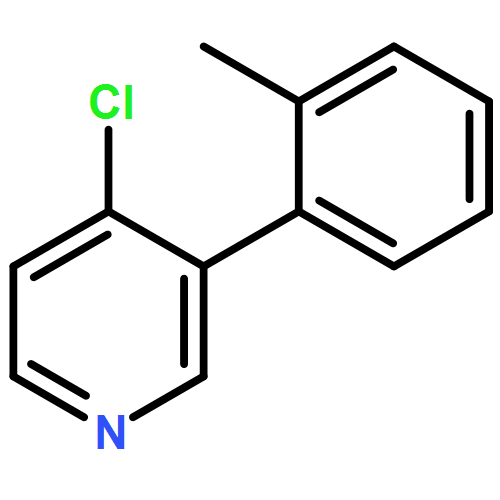
Co-reporter:Makoto Fujiwara;Masanori Sakamoto;Kimihiro Komeyama;Hiroto Yoshida
Journal of Heterocyclic Chemistry 2015 Volume 52( Issue 1) pp:59-66
Publication Date(Web):
DOI:10.1002/jhet.1964
2-Amino-4H-chromenes were synthesized in moderate to good yields by the reaction of o-quinone methides photochemically generated from o-(dimethylaminomethyl)phenols with malononitrile. This method was applicable to the synthesis of fluorinated chromenes that were difficult to obtain by other methods. In addition, o-(hydroxymethyl)phenols could be used for the reaction in the presence of tertiary amine bases.
Co-reporter:Ken Takaki, Akira Ohno, Makoto Hino, Takashi Shitaoka, Kimihiro Komeyama and Hiroto Yoshida
Chemical Communications 2014 vol. 50(Issue 82) pp:12285-12288
Publication Date(Web):26 Aug 2014
DOI:10.1039/C4CC05436A
Thiazolium carbene-catalyzed reaction of aromatic 1,2-diketones with enones in aprotic solvents gave double acylation products in good yields, whereas hydroacylation products formed by Stetter reaction were not detected at all. These results suggested the generation of aroyloxyenamine species from the 1,2-diketones instead of hydroxyenamines (Breslow intermediates).
Co-reporter:Kimihiro Komeyama, Tetsuya Kashihara, Ken Takaki
Tetrahedron Letters 2013 Volume 54(Issue 42) pp:5659-5662
Publication Date(Web):16 October 2013
DOI:10.1016/j.tetlet.2013.07.133
A cobalt-catalyzed approach for the concise synthesis of naphthalenes by the annulation of aryl iodides with alkynes is disclosed. In the reaction, manganese reductant and MeCN solvent are necessary to proceed efficiently, which tolerates various functional groups, for example, boronate ester. Mechanistic investigation indicates that the transformation employs electrophilic aromatic substitution (SEAr) in the aromatic C–H bond replacement step.
Co-reporter:Ken Takaki, Toshifumi Fujii, Hosei Yonemitsu, Makoto Fujiwara, Kimihiro Komeyama, Hiroto Yoshida
Tetrahedron Letters 2012 Volume 53(Issue 31) pp:3974-3976
Publication Date(Web):1 August 2012
DOI:10.1016/j.tetlet.2012.05.082
Hetero-Diels–Alder reaction of α-hydroxy-o-quinodimethanes photochemically generated from o-tolualdehydes with trifluoromethyl ketones gave a mixture of hemiacetals and hydroxyaldehydes in fairly good yields. Their subsequent oxidation with PCC provided 1-isochromanones as formal oxidative [4+2] cycloaddition products. In contrast, similar reaction of aromatic ketones such as o-methylbenzophenone, 1-indanone, and α-tetralone gave exclusively the corresponding ketones having (trifluoromethyl)methylol groups at the o-position.
Co-reporter:Ken Takaki, Kazuhiro Shiraishi, Kosuke Okinaga, Shintaro Takahashi and Kimihiro Komeyama
RSC Advances 2011 vol. 1(Issue 9) pp:1799-1807
Publication Date(Web):31 Oct 2011
DOI:10.1039/C1RA00482D
It has been found for the first time that the imidazole carbene-catalyzed reaction of α,β-unsaturated aldehydes with unactivated enones gives two kinds of pyranones, 1:1 and 1:2 adducts, instead of cyclopentenes and cyclopentanones. The ratio of the two products was determined by the substituent effect of the enones, not by a molar ratio of the starting materials in general. Moreover, a longer reaction period caused the formation of another 1:2 adduct, which was derived from the 1:1 pyranones initially formed.
Co-reporter:Kimihiro Komeyama Dr.;Natsuko Saigo;Motoyoshi Miyagi Dr.
Angewandte Chemie International Edition 2009 Volume 48( Issue 52) pp:9875-9878
Publication Date(Web):
DOI:10.1002/anie.200904610
Co-reporter:Kimihiro Komeyama;Takayuki Morimoto Dr.
Angewandte Chemie 2006 Volume 118(Issue 18) pp:
Publication Date(Web):23 MAR 2006
DOI:10.1002/ange.200503789
Eine neue katalytische Aktivität von Eisensalzen wird für die intramolekulare Hydroaminierung vorgestellt. Eine Reihe von Aminoolefinen reagierte dabei unter milden Bedingungen und in guten Ausbeuten zu den entsprechenden Pyrrolidinderivaten. Die Reaktion ist mit vielen funktionellen Gruppen kompatibel und nicht luft- oder feuchtigkeitsempfindlich. Ts=Tosyl.
Co-reporter:Kimihiro Komeyama;Takayuki Morimoto Dr.
Angewandte Chemie International Edition 2006 Volume 45(Issue 18) pp:
Publication Date(Web):23 MAR 2006
DOI:10.1002/anie.200503789
A new catalytic activity of iron salts for intramolecular hydroamination is revealed. A number of aminoolefins underwent the reaction under mild conditions to form the corresponding pyrrolidine derivatives in good yield. The reaction displayed good functional-group compatibility and resistance to air and moisture. (Ts=e-toluenesulfonyl)
Co-reporter:Ken Takaki, Akira Ohno, Makoto Hino, Takashi Shitaoka, Kimihiro Komeyama and Hiroto Yoshida
Chemical Communications 2014 - vol. 50(Issue 82) pp:NaN12288-12288
Publication Date(Web):2014/08/26
DOI:10.1039/C4CC05436A
Thiazolium carbene-catalyzed reaction of aromatic 1,2-diketones with enones in aprotic solvents gave double acylation products in good yields, whereas hydroacylation products formed by Stetter reaction were not detected at all. These results suggested the generation of aroyloxyenamine species from the 1,2-diketones instead of hydroxyenamines (Breslow intermediates).


![Pyridine, 4-chloro-2-[4-(4-fluorophenoxy)phenyl]-, 1-oxide](/data/chemimg/3744800/1624043-96-4.png)
![Pyridine, 4-chloro-2-[4-(4-fluorophenoxy)phenyl]-, 1-oxide](/data/chemimg/3744800/1624043-96-4_b.png)
![Pyridine, 4-chloro-3-[4-(4-fluorophenoxy)phenyl]-](/data/chemimg/3744800/1578242-00-8.png)
![Pyridine, 4-chloro-3-[4-(4-fluorophenoxy)phenyl]-](/data/chemimg/3744800/1578242-00-8_b.png)
![Pyridine, 4-chloro-2-[4-(4-fluorophenoxy)phenyl]-](/data/chemimg/3744800/1578241-99-2.png)
![Pyridine, 4-chloro-2-[4-(4-fluorophenoxy)phenyl]-](/data/chemimg/3744800/1578241-99-2_b.png)