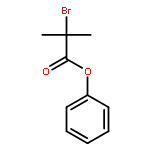
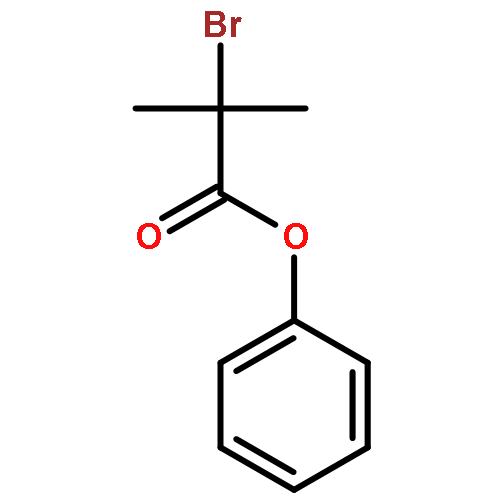
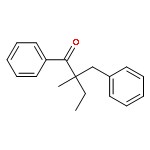
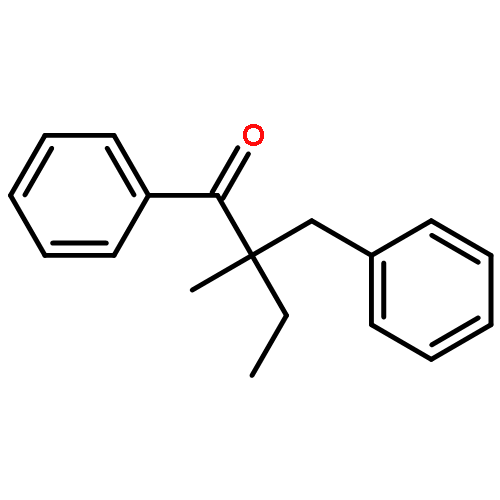
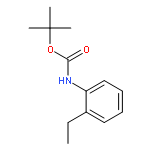
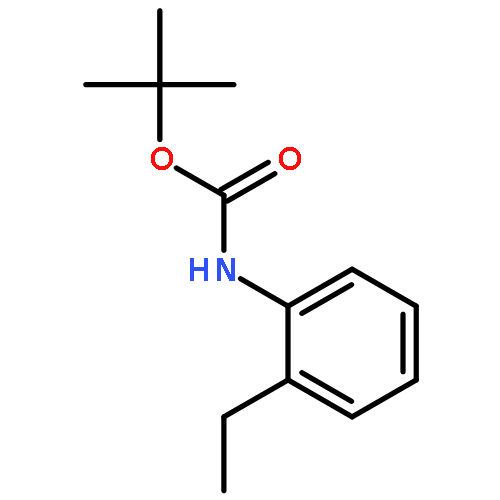
Co-reporter:Qun Li, Weipeng Hu, Renjian Hu, Hongjian Lu, and Guigen Li
Organic Letters September 1, 2017 Volume 19(Issue 17) pp:
Publication Date(Web):August 22, 2017
DOI:10.1021/acs.orglett.7b02316
Catalytic oxidative cross-dehydrogenative coupling between unactivated C(sp2)–H and C(sp3)–H bonds is achieved by the cobalt-catalyzed o-alkylation reaction of aromatic carboxamides containing (pyridin-2-yl)isopropyl amine (PIP–NH2) as a N,N-bidentate directing group. Many different C(sp3)–H bonds in alkanes, toluene derivatives and even in the α-position of ethers and thioethers can be used as coupling partners. This method has a broad substrate scope and the tolerance of various functional groups.
Co-reporter:Padmanabha V. Kattamuri, Jun Yin, Surached Siriwongsup, Doo-Hyun Kwon, Daniel H. Ess, Qun Li, Guigen Li, Muhammed Yousufuddin, Paul F. Richardson, Scott C. Sutton, and László Kürti
Journal of the American Chemical Society August 16, 2017 Volume 139(Issue 32) pp:11184-11184
Publication Date(Web):June 26, 2017
DOI:10.1021/jacs.7b05279
Given the importance of amines in a large number of biologically active natural products, active pharmaceutical ingredients, agrochemicals, and functional materials, the development of efficient C–N bond-forming methods with wide substrate scope continues to be at the frontier of research in synthetic organic chemistry. Here, we present a general and fundamentally new synthetic approach for the direct, transition-metal-free preparation of symmetrical and unsymmetrical diaryl-, arylalkyl-, and dialkylamines that relies on the facile single or double addition of readily available C-nucleophiles to the nitrogen atom of bench-stable electrophilic aminating agents. Practical single and double polarity reversal (i.e., umpolung) of the nitrogen atom is achieved using sterically and electronically tunable ketomalonate-derived imines and oximes. Overall, this novel approach represents an operationally simple, scalable, and environmentally friendly alternative to transition-metal-catalyzed C–N cross-coupling methods that are currently used to access structurally diverse secondary amines.
Co-reporter:Hongmiao Wu, Bin Yang, Lin Zhu, Ronghua Lu, Guigen Li, and Hongjian Lu
Organic Letters 2016 Volume 18(Issue 22) pp:5804-5807
Publication Date(Web):October 31, 2016
DOI:10.1021/acs.orglett.6b02706
An oxy-palladation, formal Wagner–Meerwein rearrangement and fluorination cascade has been established for generating fluorinated oxazolidine-2,4-diones and oxazolidin-2-ones. The reaction has a broad substrate scope in which both aryl and alkyl groups can be utilized as efficient migrating groups. Experimental evidence suggests that the reaction is initiated by anti-oxy-palladation of the olefin, followed by oxidative generation of an alkyl PdIV intermediate and a concerted migration–fluorination.
Co-reporter:Yu-Shun Yang, Bing Yang, Yan Zou, Guigen Li, Hai-Liang Zhu
Bioorganic & Medicinal Chemistry 2016 Volume 24(Issue 13) pp:3052-3061
Publication Date(Web):1 July 2016
DOI:10.1016/j.bmc.2016.05.012
•20 pyrazoline derivatives have been synthesized and all are novel except C1.•Their anti-cancer activities were evaluated for the first time.•Selectivity and cytotoxicity of representative compounds were tested.•Molecular docking and QSAR discussions indicated breaking the limit of previous pattern to get the most potent C6.A series of novel dioxin-containing triaryl pyrazoline derivatives C1–C20 have been synthesized. Their B-Raf inhibitory and anti-proliferation activities were evaluated. Compound C6 displayed the most potent biological activity against B-RafV600E and WM266.4 human melanoma cell line with corresponding IC50 value of 0.04 μM and GI50 value of 0.87 μM, being comparable with the positive controls and more potent than our previous best compounds. Moreover, C6 was selective for B-RafV600E from B-RafWT, C-Raf and EGFR and low toxic. The docking simulation suggested the potent bioactivity might be caused by breaking the limit of previous binding pattern. A new 3D QSAR model was built with the activity data and binding conformations to conduct visualized SAR discussion as well as to introduce new directions. Stretching the backbone to outer space or totally reversing the backbone are both potential orientations for future researches.A series of novel dioxin-containing triaryl pyrazoline derivatives C1–C20 have been synthesized with their B-Raf inhibitory activity and anti-proliferation activity tested. Selectivity and cytotoxicity of representative compounds have been tested. The docking simulation and 3D QSAR model indicated breaking the limit of previous binding pattern.
Co-reporter:Qun Li;Yanrong Li;Weipeng Hu;Renjian Hu; Guigen Li;Hongjian Lu
Chemistry - A European Journal 2016 Volume 22( Issue 35) pp:
Publication Date(Web):
DOI:10.1002/chem.201603012
Co-reporter:Qun Li;Yanrong Li;Weipeng Hu;Renjian Hu; Guigen Li;Hongjian Lu
Chemistry - A European Journal 2016 Volume 22( Issue 35) pp:12286-12289
Publication Date(Web):
DOI:10.1002/chem.201602445
Abstract
The first cobalt-catalyzed direct methylation of a C(sp2)−H bond using dicumyl peroxide (DCP) as both the methylating reagent and hydrogen acceptor has been established. The reaction proceeded without the use of any additives, and was proven to be applicable to various amides bearing a 2-pyridinylisopropyl (PIP) directing group, providing an efficient access to o-methyl aryl amides with high functional-group tolerance. Preliminary mechanistic studies suggest a radical process would be involved in the catalytic process.
Co-reporter:Guanghui An, Cole Seifert and Guigen Li
Organic & Biomolecular Chemistry 2015 vol. 13(Issue 6) pp:1600-1617
Publication Date(Web):09 Dec 2014
DOI:10.1039/C4OB02254H
The development of environmentally benign, operationally simple, and economically viable synthetic methodologies has been a great challenge in organic synthesis. Group-assisted purification (GAP) chemistry was established to enable the synthesis of organic compounds without using traditional purification technologies, such as column chromatography and recrystallization. This concept/technology should encourage the synthetic community to make more efforts on searching for environmentally benign reagents and reactions to reduce the waste generated from silica and solvents, particularly toxic solvents; also, to reduce production/synthesis expenses, manpower, and energy. This review will discuss the GAP concept/technology and related reactions that were mainly conducted in the PI's laboratories after 2010.
Co-reporter:Ke Yang, Xinyong Chen, Yuqi Wang, Wanqing Li, Adnan A. Kadi, Hoong-Kun Fun, Hao Sun, Yan Zhang, Guigen Li, and Hongjian Lu
The Journal of Organic Chemistry 2015 Volume 80(Issue 21) pp:11065-11072
Publication Date(Web):October 9, 2015
DOI:10.1021/acs.joc.5b01450
Cobalt-catalyzed decarboxylative cross-coupling of oxazoles and thiazoles with α-oxocarboxylic acids was developed through an sp2 C–H bond functionalization process. This work represents the first example of cobalt-catalyzed decarboxylative C–H bond functionalization and provides an efficient means of building some important bioactive heteroaryl ketone derivatives.
Co-reporter:Guanghui An, Cole Seifert and Guigen Li
Organic & Biomolecular Chemistry 2015 - vol. 13(Issue 6) pp:NaN1617-1617
Publication Date(Web):2014/12/09
DOI:10.1039/C4OB02254H
The development of environmentally benign, operationally simple, and economically viable synthetic methodologies has been a great challenge in organic synthesis. Group-assisted purification (GAP) chemistry was established to enable the synthesis of organic compounds without using traditional purification technologies, such as column chromatography and recrystallization. This concept/technology should encourage the synthetic community to make more efforts on searching for environmentally benign reagents and reactions to reduce the waste generated from silica and solvents, particularly toxic solvents; also, to reduce production/synthesis expenses, manpower, and energy. This review will discuss the GAP concept/technology and related reactions that were mainly conducted in the PI's laboratories after 2010.

![1-[(3,4-DIMETHOXYPHENYL)METHYL]-6,7-DIMETHOXYISOQUINOLINE;SULFURIC ACID](http://img.cochemist.com/ccimg/32900/32808-09-6.png)
![1-[(3,4-DIMETHOXYPHENYL)METHYL]-6,7-DIMETHOXYISOQUINOLINE;SULFURIC ACID](http://img.cochemist.com/ccimg/32900/32808-09-6_b.png)