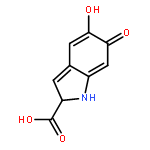
Co-reporter:Qiming Zhu, Hanmin Huang, Dengjian Shi, Zhiqiang Shen and Chungu Xia
Organic Letters 2009 Volume 11(Issue 20) pp:4536-4539
Publication Date(Web):September 10, 2009
DOI:10.1021/ol901776n
A new and efficient route for synthesis of enantiomerically pure biisoindoline and its isomer based on the diaza-Cope rearrangement reaction with chiral 1,2-bis(2-hydroxylphenyl)-1,2-diaminoethane as starting material has been developed. The newly prepared biisoindoline was employed as a chiral ligand in the Ni(II)-catalyzed enantioselective Michael addition of malonates to conjugated nitroalkenes, and good to excellent enantioselectivities were obtained.
Co-reporter:Yinjun Xie, Hanmin Huang, Weimin Mo, Xiangqun Fan, Zhiqiang Shen, Zhenlu Shen, Nan Sun, Baoxiang Hu, Xinquan Hu
Tetrahedron: Asymmetry 2009 Volume 20(Issue 12) pp:1425-1432
Publication Date(Web):2 July 2009
DOI:10.1016/j.tetasy.2009.05.014
A series of new chiral pyridine–phosphite ligands have been prepared from (R)-pyridyl alcohols and BINOL-derived chlorophosphite, and successfully employed in the copper-catalyzed enantioselective conjugate addition of diethylzinc to acyclic enones. Using the simple and inexpensive CuBr2 as a precursor, the enantioselective additions to various substituted acyclic enones afforded products in high yields and good enantioselectivities (up to 92% ee).(R)-7-O-((S)-2,2′-O,O-(1,1′-Binaphthyl)-dioxo-phosphite)-2-phenyl-6,7-dihydro-5H-cyclopenta[b]pyridineC34H25NO3PDe >99%[α]D20=+78.1 (c 0.19, CHCl3)Source of chirality: asymmetric reductionAbsolute configuration: (Rc,Sa)(R)-7-O-((R)-2,2′-O,O-(1,1′-Binaphthyl)-dioxo-phosphite)-2-phenyl-6,7-dihydro-5H-cyclopenta[b]pyridineC34H25NO3PDe >99%[α]D20=-221.5 (c 0.42, CHCl3)Source of chirality: asymmetric reductionAbsolute configuration: (Rc,Ra)(R)-7-O-((S)-2,2′-O,O-(1,1′-Binaphthyl)-dioxo-phosphite)-2-chloro-6,7-dihydro-5H-cyclopenta[b]pyridineC28H20ClNO3PDe >99%[α]D20=+231.0 (c 0.16, CHCl3)Source of chirality: asymmetric reductionAbsolute configuration: (Rc,Sa)(R)-7-O-((R)-2,2′-O,O-(1,1′-Binaphthyl)-dioxo-phosphite)-2-chloro-6,7-dihydro-5H-cyclopenta[b]pyridineC28H20ClNO3PDe >99%[α]D20=-206.1 (c 0.16, CHCl3)Source of chirality: asymmetric reductionAbsolute configuration: (Rc,Ra)(R)-8-O-((S)-2,2′-O,O-(1,1′-Binaphthyl)-dioxo-phosphite)-2-phenyl-5,6,7,8-tetrahydroquinoineC35H27NO3PDe >99%[α]D20=+72.9 (c 0.20, CHCl3)Source of chirality: asymmetric reductionAbsolute configuration: (Rc,Sa)(R)-8-O-((R)-2,2′-O,O-(1,1′-Binaphthyl)-dioxo-phosphite)-2-phenyl-5,6,7,8-tetrahydroquinoineC35H27NO3PDe >99%[α]D20=-290.7 (c 0.35, CHCl3)Source of chirality: asymmetric reductionAbsolute configuration: (Rc,Ra)(R)-2-Chloro-6,7-dihydro-5H-cyclopenta[b]pyridin-7-olC8H8ClNOEe >99%[α]D20=-4.1 (c 0.39, CHCl3)Source of chirality: asymmetric reductionAbsolute configuration: (R)




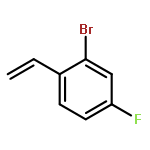
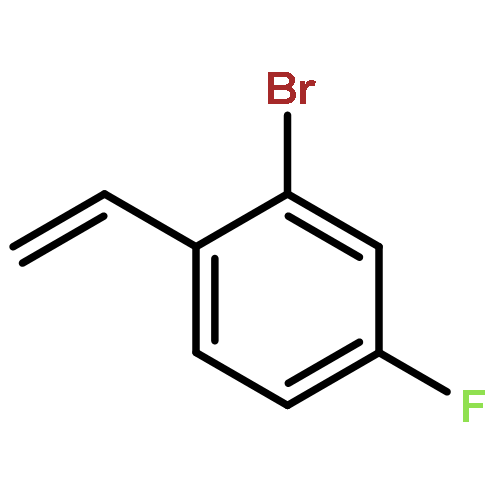




![MORPHOLINE, 4,4'-[(2-METHYLPHENYL)METHYLENE]BIS-](http://img.cochemist.com/ccimg/681000/680971-47-5.png)
![MORPHOLINE, 4,4'-[(2-METHYLPHENYL)METHYLENE]BIS-](http://img.cochemist.com/ccimg/681000/680971-47-5_b.png)
![3-Buten-1-one, 1-[4-(trifluoromethyl)phenyl]-](http://img.cochemist.com/ccimg/201200/201164-23-0.png)
![3-Buten-1-one, 1-[4-(trifluoromethyl)phenyl]-](http://img.cochemist.com/ccimg/201200/201164-23-0_b.png)


![Cyclohexanamine, N-[(2-ethenylphenyl)methylene]-](http://img.cochemist.com/ccimg/150900/150831-90-6.png)
![Cyclohexanamine, N-[(2-ethenylphenyl)methylene]-](http://img.cochemist.com/ccimg/150900/150831-90-6_b.png)