Co-reporter: Yun-Xia Hu, Hai-Bo Ma, Bo Zheng, Wen-Wei Zhang, Shengchang Xiang, Lu Zhai, Li-Feng Wang, Banglin Chen, Xiao-Ming Ren, and Junfeng Bai
pp: 7066-7074
Publication Date(Web):June 18, 2012
DOI: 10.1021/ic202085j
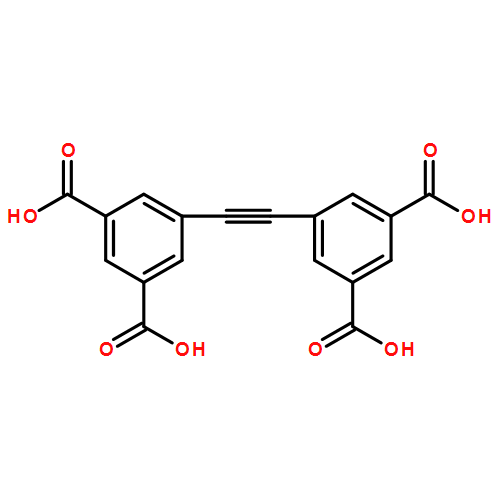
Three porous supramolecular isomers (IZE-1, IZE-2, and IZE-3) with the same framework component [Zn2(EBTC)(H2O)2] (EBTC = 1,1′-ethynebenzene-3,3′,5,5′-tetracarboxylate) were successfully constructed by finely tuning the reaction condition. Although both IZE-1 and IZE-2 are constructed from the linear EBTC subunits and one kind of regular [Zn2(CO2)4] paddlewheels, their frameworks exhibit two different (3,4)-c net of fof (sqc1575) and sqc1572, respectively, resulting in cavities with different size and shape. However, as for isomer IZE-3, the EBTC ligands are bent and one-half of the [Zn2(CO2)4] paddlewheels are distorted, leading to a novel (3,4,4)-c hyx net with point symbol (6.72)4(62.82.102)(72.82.112) and vertex symbol (6.7.7)4(72.72.8.8.12.12)(6.6.8.8.102.102). Quantum chemical calculations by DFT indicate that the three isomers have very close thermodynamic stabilities, which may explain that subtle condition change leads to variation of the frameworks. Further theoretical semiempirical investigation on the interactions between solvent molecules and compounds shows different hydrogen binding patterns in good agreement with the experimental observations. Furthermore, they exhibit good solid-state luminescence properties with long lifetime.