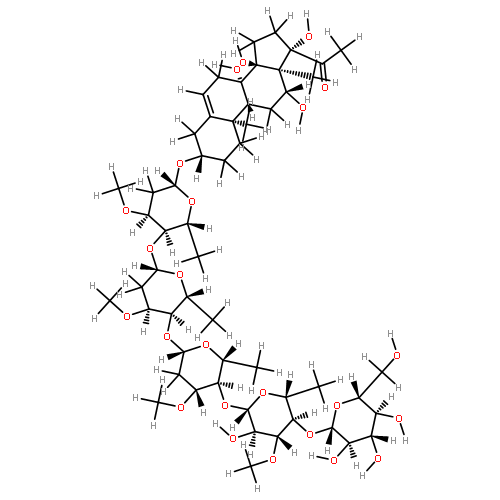
As part of our ongoing effort to explore the chemical diversity of plants of the United States Midwest region, the isolation and identification of 13 pregnane glycosides named verticillosides A–M from Asclepias verticillata L. are reported. The structures of these compounds were elucidated by various spectroscopic techniques, including 1D and 2D NMR, IR, UV, and HRMS. The cytotoxicity of the isolates was evaluated against paired breast cell lines Hs578T (cancer) and Hs578Bst (normal), however, no significant growth inhibition was observed.Graphical abstractIsolation and identification of 13 pregnane glycosides named verticillosides A–M from Asclepias verticillata L.

Highlights► Verticillosides A–M, 13 pregnane glycosides, were isolated from Asclepias verticillata L. ► 800 MHz NMR experiments and X-ray crystallography were employed for structure elucidation. ► Isolates did not show significant cytotoxicity against breast cancer cell line Hs578T.