Co-reporter: Yusuke Yonamine, Keiichi Yoshimatsu, Shih-Hui Lee, Yu Hoshino, Yoshio Okahata, and Kenneth J. Shea
pp: 374
Publication Date(Web):December 21, 2012
DOI: 10.1021/am302404q
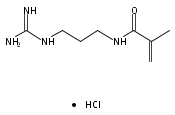
Cationic-functionalized polymer nanoparticles (NPs) show strikingly distinct affinities to proteins depending on the nature of the cationic functional group. N-Isopropylacrylamide (NIPAm) polymer NPs incorporating three types of positively charged functional groups (guanidinium, primary amino, and quaternary ammonium groups) were prepared by precipitation polymerization. The affinities to fibrinogen, a protein with an isoelectric point (pI) of 5.5, were compared using UV–vis spectrometry and a quartz crystal microbalance (QCM). Guanidinium-containing NPs showed the highest affinity to fibrinogen. The observation is attributed to strong, specific interactions with carboxylate groups on the protein surface. The affinity of the positively charged NPs to proteins with a range of pIs revealed that protein-NP affinity is due to a combination of ionic, hydrogen bonding, and hydrophobic interactions. Protein affinity can be modulated by varying the composition of these functional monomers in the acrylamide NPs. Engineered NPs containing the guanidinium group with hydrophobic and hydrogen bonding functional groups were used in an affinity precipitation for the selective separation of fibrinogen from a plasma protein mixture. Circular dichroism (CD) revealed that the protein was not denatured in the process of binding or release.Keywords: affinity precipitation; fibrinogen; guanidinium; plastic antibodies; polymer nanoparticles; protein interaction;