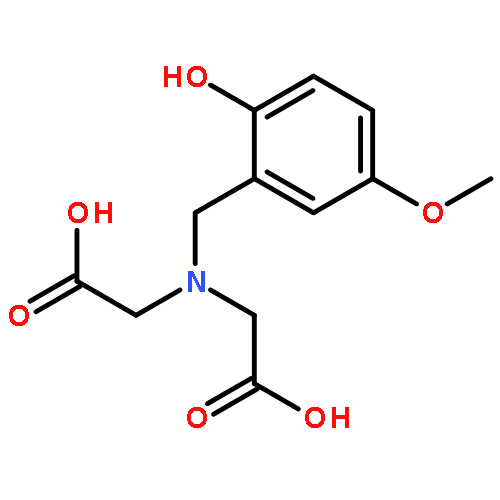
Three zirconium(IV) complexes (1–3) with mono- and dinucleating tripodal ligands are reported that promote the hydrolysis of the phosphate monoesters p-nitrophenyl phosphate (NPP) and phenyl phosphate (PP) in neutral solution. At pH 7 up to 104- and 106-fold rate accelerations of the hydrolysis of NPP and PP are observed. A detailed kinetic study has been carried out for NPP. The complexes show Michaelis–Menten behavior, kcat and 1/Km values at pH 7.0, 25 °C are 2.7 × 10−5 s−1 and 806 M−1 (1), 1.1 × 10−4 s−1 and 556 M−1 (2) and 1.1 × 10−4 s−1 and 565 M−1 (3). Entropies of activation of −92.0 J K−1 mol−1 (1), −75.6 J K−1 mol−1 (2) and −98.6 J K−1 mol−1 (3) are consistent with an associative mechanism.Graphical abstractMixtures of the mononucleating ligand L1 or of the related dinucleating ligands L2 and L3 with an excess of Zr4+ give homogeneous solutions in the pH range 6–12. The Zr complexes present in aqueous solutions promote the hydrolysis of the phosphate monoesters p-nitrophenyl phosphate and phenyl phosphate at neutral pH.
![Image for unlabelled figure]()
Highlights► Mono- and dinucleating ligands forming homogenous ZrIV solutions between pH 6 and 12. ► Rapid hydrolysis of phenyl phosphate by a dinuclear ZrIV complex at pH 7. ► Kinetic study of phosphomonoester hydrolysis by mono- and dinuclear ZrIV complexes.