Co-reporter: Jingjing Wang, Jun Zhang, Fengyan Jin, Yanping Luo, Shufen Wang, Zaiyong Zhang, Yong Wu, Hongke Liu, Jack Y. Lu and Min Fang
pp: 5906-5910
Publication Date(Web):17 Jul 2015
DOI: 10.1039/C5CE01192B
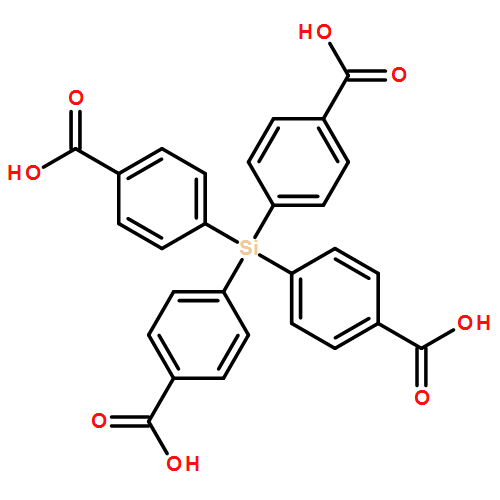
Based on the ligands tetrakis(4-carboxyphenyl)silane (TCS) and 4,4′-bipy, a novel 3D doubly interpenetrated Zn(II)-paddlewheel metal–organic framework Zn2TCS(4,4′-bipy) (1) was synthesized via facile conversion of its non-interpenetrated isomer (2) upon solvent removal at ambient temperature. It exhibits high CO2 and CH4 adsorption capacities and exceptional water stability.
Co-reporter: Shufen Wang, Jingjing Wang, Weiwei Cheng, Xiaowei Yang, Zaiyong Zhang, Yan Xu, Hongke Liu, Yong Wu and Min Fang
pp: 8049-8061
Publication Date(Web):02 Apr 2015
DOI: 10.1039/C5DT00421G
A new (4,8)-connected Zr-MOF porous zirconium metal–organic framework (Zr-MOF) with flu topology, Zr6(μ3-O)4(μ3-OH)4(TCPS)2(H2O)4(OH)4 (1, TCPS = tetrakis(4-carboxyphenyl) silane) with a BET specific area of 1402 m2 g−1 has been constructed and fully characterized. 1 is stable in air and acid media but unstable in water and basic media, and thermally stable up to 200 °C. The new MOF is a wide band gap semiconductor with Eg = 3.95 eV. The excitation of 1 at 260 nm gives a ligand-based emission peak at 435 nm. After solvent exchange processes and activation at 200 °C, this MOF exhibits high storage capacities for H2, CH4 and CO2. We summarized the hydrothermal stability data of Zr-MOFs, calculated the NBO (natural bond orbital) charges of the coordinating oxygen atoms of the corresponding carboxylate ligands and analyzed the influencing factors. Besides the known reasons of hydrothermal stabilities of Zr-MOFs, we demonstrated that NBO charges of coordinating atoms of the ligands can be used to explain the hydrothermal stabilities of Zr-MOFs.
Co-reporter: Shufen Wang, Jingjing Wang, Weiwei Cheng, Xiaowei Yang, Zaiyong Zhang, Yan Xu, Hongke Liu, Yong Wu and Min Fang
pp: NaN8061-8061
Publication Date(Web):2015/04/02
DOI: 10.1039/C5DT00421G
A new (4,8)-connected Zr-MOF porous zirconium metal–organic framework (Zr-MOF) with flu topology, Zr6(μ3-O)4(μ3-OH)4(TCPS)2(H2O)4(OH)4 (1, TCPS = tetrakis(4-carboxyphenyl) silane) with a BET specific area of 1402 m2 g−1 has been constructed and fully characterized. 1 is stable in air and acid media but unstable in water and basic media, and thermally stable up to 200 °C. The new MOF is a wide band gap semiconductor with Eg = 3.95 eV. The excitation of 1 at 260 nm gives a ligand-based emission peak at 435 nm. After solvent exchange processes and activation at 200 °C, this MOF exhibits high storage capacities for H2, CH4 and CO2. We summarized the hydrothermal stability data of Zr-MOFs, calculated the NBO (natural bond orbital) charges of the coordinating oxygen atoms of the corresponding carboxylate ligands and analyzed the influencing factors. Besides the known reasons of hydrothermal stabilities of Zr-MOFs, we demonstrated that NBO charges of coordinating atoms of the ligands can be used to explain the hydrothermal stabilities of Zr-MOFs.