Easily accessible diterpenoid alkaloid derivatives have been used as organocatalysts in the enantioselective α-chlorination of β-oxo esters. The treatment of β-oxo esters with N-chlorophthalimide (NCP) as a chlorine source under mild reaction conditions afforded the corresponding α-chlorinated β-oxo esters in excellent yields (up to 98%) and with moderate enantioselectivities (up to 68% ee) in 30 min.


LappaconitineC32H44N2O8Source of chirality: natural occurrenceAbsolute configuration: (1S,4S,5S,7S,8S,9S,10S,11S,13R,14S,16S,17S)[α]D20 = +29.25 (c 0.40, MeOH)

VincamineC21H26N2O3Source of chirality: natural occurrenceAbsolute configuration: (3S,14S,16S)[α]D20 = +11.5 (c 0.51, CH2Cl2)

VinpocetineC21H24N2O2Source of chirality: natural occurrenceAbsolute configuration: (3S,16S)[α]D20 = +130.3 (c 0.50, CH2Cl2)

CytisineC11H14N2OSource of chirality: natural occurrenceAbsolute configuration: (1R,5S)[α]D20 = −111.9 (c 0.51, MeOH)

GalantamineC17H21NO3Source of chirality: natural occurrenceAbsolute configuration: (4αS,6R,8αS)[α]D20 = −117.0 (c 0.49, MeOH)

SinomenineC19H23NO4Source of chirality: natural occurrenceAbsolute configuration: (9S,13R,14S)[α]D20 = −87.2 (c 0.52, MeOH)

N-DeethyllappaconitineC30H40N2O8Source of chirality: lappaconitineAbsolute configuration: (1S,4S,5S,7S,8S,9S,10S,11S,13R,14S,16S,17S)[α]D20 = +42.7 (c 0.52, MeOH)

N-Methyl-N-deethyllappaconitineC31H42N2O8Source of chirality: lappaconitineAbsolute configuration: (1S,4S,5S,7S,8S,9S,10S,11S,13R,14S,16S,17S)[α]D20 = +27.2 (c 0.50, MeOH)

N-Benzyl-N-deethyllappaconitineC37H46N2O8Source of chirality: lappaconitineAbsolute configuration: (1S,4S,5S,7S,8S,9S,10S,11S,13R,14S,16S,17S)[α]D20 = +14.95 (c 0.50, MeOH)

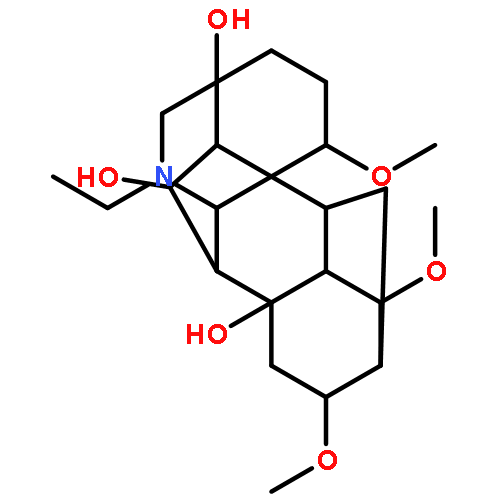
LappaconineC23H37NO6Source of chirality: lappaconitineAbsolute configuration: (1S,4S,5S,7S,8S,9S,10S,11R,13R,14S,16S,17S)[α]D20 = +15.45 (c 0.51, MeOH)

4-BenzyloxylappaconineC30H43NO6Source of chirality: lappaconineAbsolute configuration: (1S,4S,5S,7S,8S,9S,10S,11S,13R,14S,16S,17S)[α]D20 = +2.3 (c 0.49, MeOH)

8,9-(Methylenedioxy)lappaconineC24H37NO6Source of chirality: lappaconineAbsolute configuration: (1S,4S,5S,7S,8S,9S,10S,11R,13R,14S,16S,17S)[α]D20 = +13.6 (c 0.50, CHCl3)