Co-reporter: Junpeng Liu, Qinghua Zhang, Qingming Xia, Jie Dong, Qian Xu
pp: 987-994
Publication Date(Web):June 2012
DOI: 10.1016/j.polymdegradstab.2012.03.010
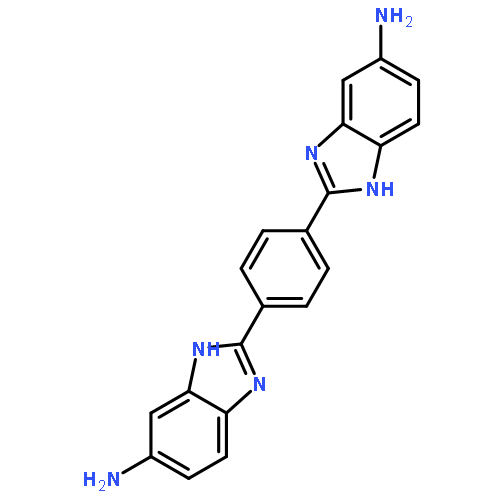
A new aromatic heterocyclic diamine monomer containing bis-benzimidazole rings, 2,2′-p-phenylene-bis(5-aminobenzimidazole) (PBABI), was synthesized from 2,2′-p-phenylene-bis (5-nitrobenzimidazole) with high yield via the reaction between 4-nitro-1,2-phenylenediamine and terephthalyl chloride. The composition and structure of the resulting diamine monomer were studied by means of FTIR, 1H and 13C NMR and elemental analysis. A series of polyimides holding bis-benzimidazole rings in main chain were produced by reacting PBABI with the aromatic dianhydrides 3,3′,4,4′-biphenyl tetracarboxylic dianhydride, 4,4′-oxydiphthalic anhydride, benzophenone tetracarboxylic dianhydride and pyromellitic dianhydride via a conventional, two-step procedure. X-ray diffraction and differential scanning calorimetry were employed to investigate the polyimides, revealing them essentially amorphous. The polyimides possess thermal stabilities of up to 560 °C for a 5% weight loss and glass transition temperatures above 450 °C. In addition, these high performance polymers have excellent mechanical properties, exhibiting tensile strengths of 120–180 MPa and tensile moduli of 4.83–5.79 GPa without any stretching. The rigid-rod structure of polyimides and the hydrogen bonding of their inter-macromolecular chains are most likely responsible for the outstanding properties of the polyimides.