BASIC PARAMETERS Find an error
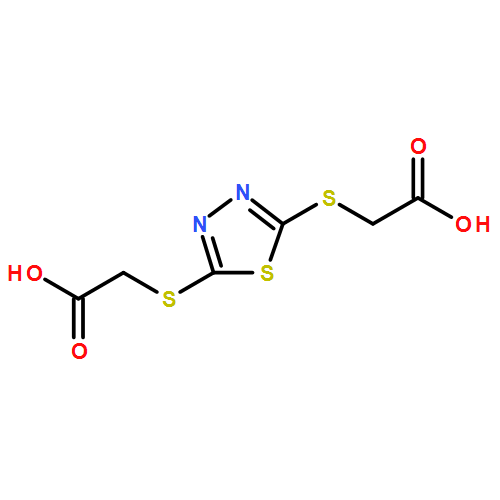
Three series of copper–lanthanide/lanthanide coordination polymers (CPs) LnIIICuIICuI(bct)3(H2O)2 [Ln=La (1), Ce (2), Pr (3), Nd (4), Sm (5), Eu (6), Gd (7), Tb (8), Dy (9), Er (10), Yb (11), and Lu (12), H2bct=2,5-bis(carboxymethylmercapto)-1,3,4-thiadiazole acid], LnIIICuI(bct)2 [Ln=Ce (2 a), Pr (3 a), Nd (4 a), Sm (5 a), Eu (6 a), Gd (7 a), Tb (8 a), Dy (9 a), Er (10 a), Yb (11 a), and Lu (12 a)], and LnIII2(bct)3(H2O)5 [Ln=La (1 b), Ce (2 b), Pr (3 b), Nd (4 b), Sm (5 b), Eu (6 b), Gd (7 b), Tb (8 b), and Dy (9 b)] have been successfully constructed under hydrothermal conditions by modulating the reaction time. Structural characterization has revealed that CPs 1–12 possess a unique one-dimensional (1D) strip-shaped structure containing two types of double-helical chains and a double-helical channel. CPs 2 a–12 a show a three-dimensional (3D) framework formed by CuI linking two types of homochiral layers with double-helical channels. CPs 1 b–9 b exhibit a 3D framework with single-helical channels. CPs 6 b and 8 b display visible red and green luminescence of the EuIII and TbIII ions, respectively, sensitized by the bct ligand, and microsecond-level lifetimes. CP 8 b shows a rare magnetic transition between short-range ferromagnetic ordering at 110 K and long-range ferromagnetic ordering below 10 K. CPs 9 a and 9 b display field-induced single-chain magnet (SCM) and/or single-molecule magnet (SMM) behaviors, with Ueff values of 51.7 and 36.5 K, respectively.
The observed structure of 1,3,4-thiadiazolidine-2,5-dithione (also known as 2,5-dimercapto-1,3,4-thiadiazole) has been previously reported in three different tautomeric forms including —dithiol and—dithione. This report examines the relative stability of each form and also reports synthesis and characterization of the structures of mono-alkylated and di-alkylated forms of 5-mercapto-1,3,4-thiadiazole-2(3H)-thione. The methods of X-ray crystallography, NMR spectroscopy, and ab initio electronic structure calculations were combined to understand the reactivity and structure of each compound.
The observed structure of 1,3,4-thiadiazolidine-2,5-dithione (also known as 2,5-dimercapto-1,3,4-thiadiazole) has been previously reported in three different tautomeric forms including —dithiol and—dithione. This report examines the relative stability of each form and also reports synthesis and characterization of the structures of mono-alkylated and di-alkylated forms of 5-mercapto-1,3,4-thiadiazole-2(3H)-thione. The methods of X-ray crystallography, NMR spectroscopy, and ab initio electronic structure calculations were combined to understand the reactivity and structure of each compound.