Abstract
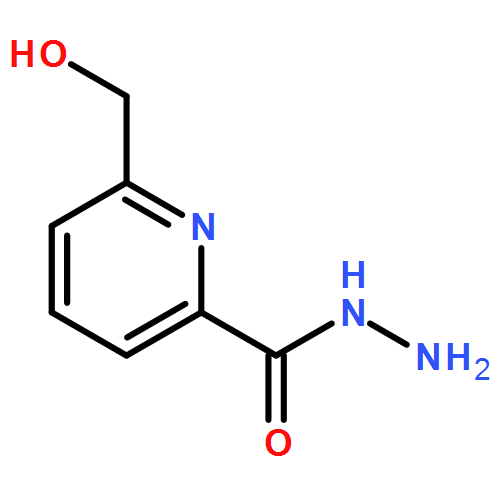
The reactions of lanthanide(III) nitrate salts (DyIII, TbIII, GdIII, and ErIII) with the aroylhydrazone-based multidentate ligand 6-(hydroxymethyl)-N′-[1-(pyridin-2-yl)ethylidene]picolinohydrazide (LH2) in the presence of Et3N in a molar ratio of 1:1:4 afforded a series of homometallic tetranuclear lanthanide(III) complexes, [Ln4(LH)4(μ2-OH)3(μ2-OMe)]4NO3·xMeOH·yH2O (1, Ln = Dy, x = 2, y = 4; 2, Ln = Tb, x = 2, y = 4; 3, Ln = Gd, x = 2, y = 5; and 4, Ln = Er, x = 3, y = 3). X-ray diffraction studies revealed that all of the complexes contain a tetracationic [2×2] square-grid-like [Ln4(μ2-OH)3(μ2-OMe)(μ2-O)4]4+ core, which is assembled by the concerted coordination action of four monoanionic [LH]– ligands along with three μ2-OH ligands and a μ2-OMe ligand. All of the lanthanide centers are eight-coordinate and adopt distorted triangular-dodecahedral coordination geometries with two different types of coordination environments (6O,2N and 4O,4N). The magnetic susceptibility measurements of the complexes reveal both the presence of all-antiferromagnetic coupling interactions as well as both isotropic (3) and anisotropic (1, 2, 4) single-ion contributions, which do not result in slow relaxation characteristics typical of single-molecule magnets.