Co-reporter: Zhe Rui, Wei Huang, Fei Xu, Mo Han, Xinyu Liu, Shuangjun Lin, and Wenjun Zhang
pp: 1765
Publication Date(Web):June 5, 2015
DOI: 10.1021/acschembio.5b00284
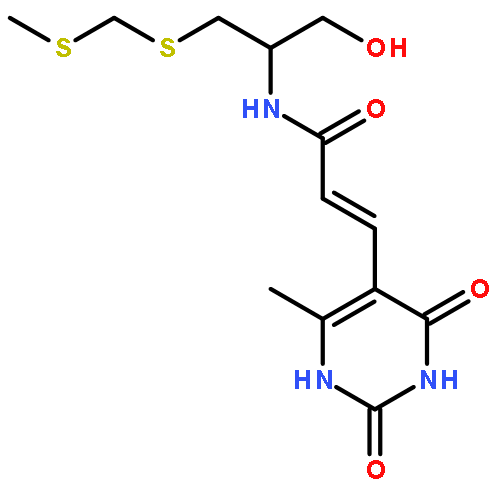
Sparsomycin is a model protein synthesis inhibitor that blocks peptide bond formation by binding to the large ribosome subunit. It is a unique dipeptidyl alcohol, consisting of a uracil acrylic acid moiety and a monooxo-dithioacetal group. To elucidate the biosynthetic logic of sparsomycin, a biosynthetic gene cluster for sparsomycin was identified from the producer Streptomyces sparsogenes by genome mining, targeted gene mutations, and heterologous expression. Both the genetic and enzymatic studies revealed a minimum set of non-ribosomal peptide synthetases needed for generating the dipeptidyl alcohol scaffold of sparsomycin, featuring unusual mechanisms in dipeptidyl assembly and off-loading.