Co-reporter:Jing Hu, Tian-Ming Wu, Hong-Ze Li, Ze-Ping Zuo, Ying-Lan Zhao, Li Yang
Bioorganic & Medicinal Chemistry Letters 2017 Volume 27, Issue 15(Issue 15) pp:
Publication Date(Web):1 August 2017
DOI:10.1016/j.bmcl.2017.04.077
Cisplatin is a widely used antineoplastic drug, while its nephrotoxicity limits the clinical application. Although several mechanisms contributing to nephrotoxicity have been reported, the direct protein targets are unclear. Herein we reported the synthesis of 29 cisplatin derivatives and the structure-toxicity relationship (STR) of these compounds with MTT assay in human renal proximal tubule cells (HK-2) and pig kidney epithelial cells (LLC-PK1). To the best of our knowledge, this study represented the first report regarding the structure-toxicity relationship (STR) of cisplatin derivatives. The potency of biotin-pyridine conjugated derivative 3 met the requirement for target identification, and the preliminary chemical proteomics results suggested that it is a promising tool for further target identification of cisplatin-induced nephrotoxicity.Herein we reported the synthesis of 29 cisplatin derivatives and the structure-toxicity relationship (STR) of these compounds with MTT assay. The potency of biotin-pyridine conjugated derivative 3 met the requirement for target identification, and the preliminary chemical proteomics results suggested that it is a promising tool for further target identification of cisplatin-induced nephrotoxicity.Download high-res image (74KB)Download full-size image
Co-reporter:Huan Liu, Yi Li, Xiang-Ying Wang, Bo Wang, Hai-Yun He, Ji-Yan Liu, Ming-Li Xiang, Jun He, Xiao-Hua Wu, Li Yang
Bioorganic & Medicinal Chemistry Letters 2013 Volume 23(Issue 8) pp:2349-2352
Publication Date(Web):15 April 2013
DOI:10.1016/j.bmcl.2013.02.059
In our previous study, a series of 6-aryl-3-amino-thieno[2,3-b]pyridine derivatives exhibited potent antiproliferative activities and an unique hepatocellular carcinoma (HCC)-specific anticancer activity was also observed. In further anti-inflammatory research, thienopyridine derivative 1a showed potent inhibition of nitric oxide (NO) production. So a series of thienopyridine analogues of 1a were synthesized and evaluated for anti-inflammatory activities. The structure–activity relationships (SARs) revealed that the most potent analogues 1f and 1o were identified as potent inhibitors of NO production with IC50 values of 3.30 and 3.24 μM, respectively. These results suggest that these 6-aryl-3-amino-thieno[2,3-b]pyridine derivatives might potentially constitute a novel class of anti-inflammatory agents, which require further studies.To the best of our knowledge, the anti-inflammatory activity of 6-aryl-3-amino-thieno[2,3-b]pyridine derivative is being firstly reported by our group.
Co-reporter:Xiu-Xiu Zeng, Ren-Lin Zheng, Tian Zhou, Hai-Yun He, Ji-Yan Liu, Yu Zheng, Ai-Ping Tong, Ming-Li Xiang, Xiang-Rong Song, Sheng-Yong Yang, Luo-Ting Yu, Yu-Quan Wei, Ying-Lan Zhao, Li Yang
Bioorganic & Medicinal Chemistry Letters 2010 Volume 20(Issue 21) pp:6282-6285
Publication Date(Web):1 November 2010
DOI:10.1016/j.bmcl.2010.08.088
Novel thienopyridine derivatives 1b–1r were synthesized, based on a hit compound 1a that was found in a previous cell-based screening of anticancer drugs. Compounds 1a–1r have the following features: (1) their anticancer activity in vitro was first reported by our group. (2) The most potent analog 1g possesses hepatocellular carcinoma (HCC)-specific anticancer activity. It can specifically inhibit the proliferation of the human hepatoma HepG2 cells with an IC50 value of 0.016 μM (compared with doxorubicin as a positive control, whose IC50 was 0.37 μM). It is inactive toward a panel of five different types of human cancer cell lines. (3) Compound 1g remarkably induces G0/G1 arrest and apoptosis in HepG2 cells in vitro at low micromolar concentrations. These results, especially the HCC-specific anticancer activity of 1g, suggest their potential in targeted chemotherapy for HCC.Among 17 analogs of 1a, the most potent analog 1g possesses hepatocellular carcinoma (HCC)-specific anticancer activity. It was inactive toward a panel of five different types of human cancer cell lines.












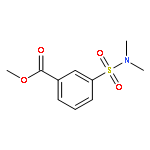
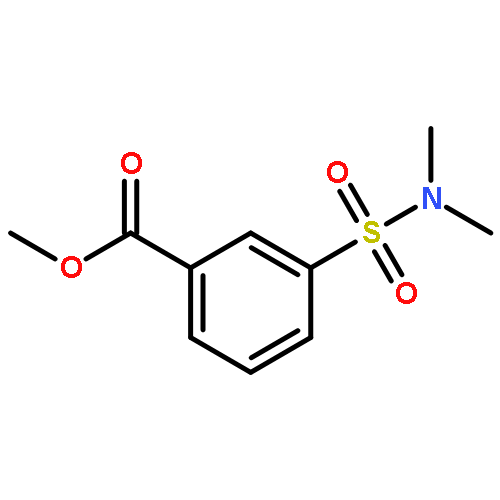
![Benzoic acid, 3-[(phenylamino)sulfonyl]-, methyl ester](http://img.cochemist.com/ccimg/866400/866324-02-9.png)
![Benzoic acid, 3-[(phenylamino)sulfonyl]-, methyl ester](http://img.cochemist.com/ccimg/866400/866324-02-9_b.png)