Co-reporter:Zenghua Chen;Le’an Hu;Fanyun Zeng;Ranran Zhu;Shasha Zheng;Qingzhen Yu
Chemical Communications 2017 vol. 53(Issue 30) pp:4258-4261
Publication Date(Web):2017/04/11
DOI:10.1039/C7CC01201B
We report our latest discovery of norbornene derivative modulated highly mono-selective ortho-C–H activation alkylation reactions on arenes bearing simple mono-dentate coordinating groups. The reaction features the use of readily available benzamides and alkyl halides. During the study, we prepared 30 mono-alkylated aryl amides in good yields with good mono-selectivity. We have also demonstrated that structurally rigid alkenes such as norbornene and its derivatives are a good class of ligand and could be used for future direct C–H functionalizations. The utilization of norbornene type ligands for assistance in C–H activation processes has opened a new window for future molecular design using direct C–H functionalization strategies.
Co-reporter:Yuntao Wang;Jialin Liu;Lin Huang;Ranran Zhu;Xueyan Huang;Ross Moir
Chemical Communications 2017 vol. 53(Issue 33) pp:4589-4592
Publication Date(Web):2017/04/20
DOI:10.1039/C7CC02006F
A practical preparation of the reagent PMDTALi using a super base system under mild conditions has been developed. This PMDTALi base has been demonstrated to be a very efficient reagent for the lithiation of bridged alkenes via direct deprotonation. Further reactions with electrophiles and also coupling reactions in the presence of Pd catalysts provide the bridged alkenes with a broad range of functional groups including silyl, alkyl, halogen and aryl substituents. The utilization of this new lithium reagent has brought a new diversity to the choice of lithium reagent for the deprotonation of synthetically challenging systems.
Co-reporter:Weidong Liu, Qingzhen Yu, Le'an Hu, Zenghua Chen and Jianhui Huang
Chemical Science 2015 vol. 6(Issue 10) pp:5768-5772
Publication Date(Web):26 Jun 2015
DOI:10.1039/C5SC01482D
An efficient synthesis of dihydro-isoquinolines via a Pd–catalyzed double C–H bond [a C(sp2)–H and a C(sp3)–H bond] activation/annulation (CHAA) reaction is presented. This methodology features a short reaction time, high atom economy (loss of H2O only) and the formation of a sterically less favoured tertiary C–N bond. This fast (30 min) and environmentally benign radical C–H activation approach has demonstrated the potential direction for the future design/development of fast and efficient C–H direct functionalization processes.
Co-reporter:Dr. Qingzhen Yu;Le'an Hu;Yue Wang;Shasha Zheng;Dr. Jianhui Huang
Angewandte Chemie 2015 Volume 127( Issue 50) pp:15499-15503
Publication Date(Web):
DOI:10.1002/ange.201507100
Abstract
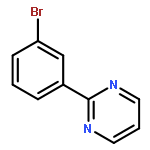
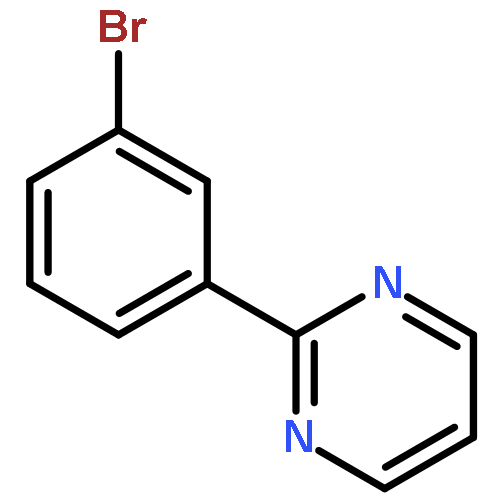
A Ru-catalyzed direct CH activation/meta-bromination of arenes bearing pyridyl, pyrimidyl, and pyrazolyl directing groups has been developed. A series of bromo aryl pyridines and pyrimidines have been synthesized, and further coupling reactions have also been demonstrated for a number of representative functionalized arenes. Preliminary mechanistic studies have revealed that this reaction may proceed through radical-mediated bromination when NBS is utilized as the bromine source. This type of transformation has opened up a new direction for the radical non-ipso functionalization of metal with regard to future CH activation development that would allow the remote functionalization of aromatic systems.
Co-reporter:Dr. Qingzhen Yu;Le'an Hu;Yue Wang;Shasha Zheng;Dr. Jianhui Huang
Angewandte Chemie International Edition 2015 Volume 54( Issue 50) pp:15284-15288
Publication Date(Web):
DOI:10.1002/anie.201507100
Abstract
A Ru-catalyzed direct CH activation/meta-bromination of arenes bearing pyridyl, pyrimidyl, and pyrazolyl directing groups has been developed. A series of bromo aryl pyridines and pyrimidines have been synthesized, and further coupling reactions have also been demonstrated for a number of representative functionalized arenes. Preliminary mechanistic studies have revealed that this reaction may proceed through radical-mediated bromination when NBS is utilized as the bromine source. This type of transformation has opened up a new direction for the radical non-ipso functionalization of metal with regard to future CH activation development that would allow the remote functionalization of aromatic systems.
Co-reporter:Nana Zhang, Qingzhen Yu, Ruixue Chen, Jianhui Huang, Yeqing Xia and Kang Zhao
Chemical Communications 2013 vol. 49(Issue 82) pp:9464-9466
Publication Date(Web):14 Aug 2013
DOI:10.1039/C3CC45449E
A novel Pd-catalysed C–H activation reaction for the synthesis of biaryl imino/keto carboxylic acids is developed. This reaction underwent aryl amide directed C–H activation ortho-acylation followed by ring closing and ring opening processes to give a range of biaryl imino/keto carboxylic acids. Our methodology features the utilization of a cheap and green oxidant (TBHP) as well as readily available aldehydes.
Co-reporter:Shaonan Lu, Yingfu Lin, Hongban Zhong, Kang Zhao, Jianhui Huang
Tetrahedron Letters 2013 Volume 54(Issue 15) pp:2001-2005
Publication Date(Web):10 April 2013
DOI:10.1016/j.tetlet.2013.02.003
A practical one-pot procedure for the preparation of N–H isoquinolones has been developed. This 2-step process via C–H activation of N-alkoxyl benzamides and NaH-mediated dealkoxylation reaction has been demonstrated to be a high yielding alternative methodology for the efficient synthesis of a wide range of representative N–H isoquiolones. In addition, the experimental precedent supported mechanism of the dealkoxylation reaction was also proposed.
Co-reporter:Qingzhen Yu;Nana Zhang;Dr. Jianhui Huang;Shaonan Lu;Yi Zhu;Xiaoxiao Yu;Dr. Kang Zhao
Chemistry - A European Journal 2013 Volume 19( Issue 34) pp:11184-11188
Publication Date(Web):
DOI:10.1002/chem.201302031
Co-reporter:Hongban Zhong, Dan Yang, Songqing Wang and Jianhui Huang
Chemical Communications 2012 vol. 48(Issue 26) pp:3236-3238
Publication Date(Web):01 Feb 2012
DOI:10.1039/C2CC17859A
An atom economical synthesis of isoquinolinones and analogues via ligand-free Pd-catalysed C–H and N–H double activation has been developed. A series of isoquinolinones were obtained in good to excellent yields. Good regioselectivities were also observed during the activation reactions with unsymmetrical alkynes. A practical one-pot procedure for the preparation of N–H isoquinolinones is also described.
Co-reporter:Nana Zhang, Binyao Li, Hongban Zhong and Jianhui Huang
Organic & Biomolecular Chemistry 2012 vol. 10(Issue 47) pp:9429-9439
Publication Date(Web):09 Oct 2012
DOI:10.1039/C2OB26668G
Relatively less expensive Pd-catalysed oxidative coupling reactions for the preparation of N-alkyl and N-aryl substituted isoquinolones and their derivatives have been developed. A broad reaction scope has been demonstrated. In addition, studies of the reaction additives and mechanistic insights, as well as KIE studies for a better understanding of the reaction pathway, have been discussed.
Co-reporter:Le Liu, Yanfeng Fan, Qiaoqiao He, Yun Zhang, Daisy Zhang-Negrerie, Jianhui Huang, Yunfei Du, and Kang Zhao
The Journal of Organic Chemistry 2012 Volume 77(Issue 8) pp:3997-4004
Publication Date(Web):March 28, 2012
DOI:10.1021/jo300367q
A series of functionalized indenes bearing 1,3-dicyano groups were synthesized from electron-rich α-aryl ketonitriles in the presence of K3Fe(CN)6 and NaOAc, possibly through tandem process involving dimerization, heterolytic cleavage of carbon–carbon bond, intermolecular coupling, and the subsequent intramolecular cyclization. The 2-arylindene compounds obtained possess good fluorescent properties.
Co-reporter:Weidong Liu, Qingzhen Yu, Le'an Hu, Zenghua Chen and Jianhui Huang
Chemical Science (2010-Present) 2015 - vol. 6(Issue 10) pp:NaN5772-5772
Publication Date(Web):2015/06/26
DOI:10.1039/C5SC01482D
An efficient synthesis of dihydro-isoquinolines via a Pd–catalyzed double C–H bond [a C(sp2)–H and a C(sp3)–H bond] activation/annulation (CHAA) reaction is presented. This methodology features a short reaction time, high atom economy (loss of H2O only) and the formation of a sterically less favoured tertiary C–N bond. This fast (30 min) and environmentally benign radical C–H activation approach has demonstrated the potential direction for the future design/development of fast and efficient C–H direct functionalization processes.
Co-reporter:Nana Zhang, Binyao Li, Hongban Zhong and Jianhui Huang
Organic & Biomolecular Chemistry 2012 - vol. 10(Issue 47) pp:NaN9439-9439
Publication Date(Web):2012/10/09
DOI:10.1039/C2OB26668G
Relatively less expensive Pd-catalysed oxidative coupling reactions for the preparation of N-alkyl and N-aryl substituted isoquinolones and their derivatives have been developed. A broad reaction scope has been demonstrated. In addition, studies of the reaction additives and mechanistic insights, as well as KIE studies for a better understanding of the reaction pathway, have been discussed.
Co-reporter:Hongban Zhong, Dan Yang, Songqing Wang and Jianhui Huang
Chemical Communications 2012 - vol. 48(Issue 26) pp:NaN3238-3238
Publication Date(Web):2012/02/01
DOI:10.1039/C2CC17859A
An atom economical synthesis of isoquinolinones and analogues via ligand-free Pd-catalysed C–H and N–H double activation has been developed. A series of isoquinolinones were obtained in good to excellent yields. Good regioselectivities were also observed during the activation reactions with unsymmetrical alkynes. A practical one-pot procedure for the preparation of N–H isoquinolinones is also described.
Co-reporter:Nana Zhang, Qingzhen Yu, Ruixue Chen, Jianhui Huang, Yeqing Xia and Kang Zhao
Chemical Communications 2013 - vol. 49(Issue 82) pp:NaN9466-9466
Publication Date(Web):2013/08/14
DOI:10.1039/C3CC45449E
A novel Pd-catalysed C–H activation reaction for the synthesis of biaryl imino/keto carboxylic acids is developed. This reaction underwent aryl amide directed C–H activation ortho-acylation followed by ring closing and ring opening processes to give a range of biaryl imino/keto carboxylic acids. Our methodology features the utilization of a cheap and green oxidant (TBHP) as well as readily available aldehydes.
Co-reporter:Yuntao Wang, Jialin Liu, Lin Huang, Ranran Zhu, Xueyan Huang, Ross Moir and Jianhui Huang
Chemical Communications 2017 - vol. 53(Issue 33) pp:NaN4592-4592
Publication Date(Web):2017/03/28
DOI:10.1039/C7CC02006F
A practical preparation of the reagent PMDTALi using a super base system under mild conditions has been developed. This PMDTALi base has been demonstrated to be a very efficient reagent for the lithiation of bridged alkenes via direct deprotonation. Further reactions with electrophiles and also coupling reactions in the presence of Pd catalysts provide the bridged alkenes with a broad range of functional groups including silyl, alkyl, halogen and aryl substituents. The utilization of this new lithium reagent has brought a new diversity to the choice of lithium reagent for the deprotonation of synthetically challenging systems.
Co-reporter:Zenghua Chen, Le’an Hu, Fanyun Zeng, Ranran Zhu, Shasha Zheng, Qingzhen Yu and Jianhui Huang
Chemical Communications 2017 - vol. 53(Issue 30) pp:NaN4261-4261
Publication Date(Web):2017/03/15
DOI:10.1039/C7CC01201B
We report our latest discovery of norbornene derivative modulated highly mono-selective ortho-C–H activation alkylation reactions on arenes bearing simple mono-dentate coordinating groups. The reaction features the use of readily available benzamides and alkyl halides. During the study, we prepared 30 mono-alkylated aryl amides in good yields with good mono-selectivity. We have also demonstrated that structurally rigid alkenes such as norbornene and its derivatives are a good class of ligand and could be used for future direct C–H functionalizations. The utilization of norbornene type ligands for assistance in C–H activation processes has opened a new window for future molecular design using direct C–H functionalization strategies.


![Thieno[2,3-c]pyridin-7(6H)-one, 4,5-diphenyl-](/data/chemimg/141000/1360651-35-9.png)
![Thieno[2,3-c]pyridin-7(6H)-one, 4,5-diphenyl-](/data/chemimg/141000/1360651-35-9_b.png)