
The phototropin from Chlamydomonas reinhardtii is a 120 kDa blue light receptor that plays a key role in gametogenesis of this green alga. It comprises two light-sensing domains termed LOV1 and LOV2 (light oxygen and voltage) and a serine/threonine kinase domain. The post-translationally incorporated chromophore is flavin mononucleotide (FMN). Upon absorption of blue light, LOV domains undergo a photocycle that activates a Ser/Thr kinase. The mechanism of this activation is still unknown. We studied the oligomerization of the recombinant LOV1 domain (amino acids 16–133) of C. reinhardtii by means of UV/Vis spectroscopy, size-exclusion chromatography (SEC), and chemical cross-linking with glutardialdehyde. The thermal back-reaction of LOV1 from the signaling state to the dark state as monitored by UV/Vis spectroscopy after an intensive blue light pulse could not be explained by a monoexponential model, although the spectra did not indicate the presence of an additional species. Therefore, we investigated the quaternary structure of the LOV1 domain by size-exclusion chromatography in the dark. This revealed an equilibrium between dimers and higher oligomers (MW>200 kDa) under native conditions. No monomers were detected by SEC. However, by analysis of the equilibrium by cross-linking of the protein with glutardialdehyde and subsequent SDS-PAGE, monomers and dimers were identified. Exposure of LOV1 to blue light resulted in a decrease in the monomer/dimer ratio, followed by re-equilibration in the dark. Calculation of the solvent-accessible surface area and the Conolly surfaces of the LOV1 dimers present in the crystal structure support the experimental observation that no mononomers are detected in the native state. A model is presented that accounts for a blue-light-driven change in the quaternary structure of the LOV1 domain and gives hints to the molecular basis of light activation and regulation in LOV-containing proteins.
The establishment of two new breast cancer cell lines, MXT+ and MXT−, derived from the murine breast cancer models MXT-M-3, 2 MC (hormone-sensitive) and MXT-M-3, 2 (ovex) MC (hormone-insensitive), is described. Characterization of the cell lines was performed by investigation of morphology, steroid hormone receptor state, growth kinetics, and drug response as well as by cytogenetic analysis. MXT+ contains estrogen receptors (ER; 6.9 fmol/mg protein) as well as progesterone receptors (PgR; 9.2 fmol/mg protein) and therefore is inhibited by tamoxifen (Tam). MXT− proved to be ER− but PgR+ (23.4 fmol/mg protein) and, as expected, resistant against Tam.The sensitivity of MXT+ and MXT− against a pattern of therapeutically established anti-breast cancer drugs (cDDP, cisplatin; JM-8, carboplatin; DX, adriamycin; 5-FU, 5-fluorouracil; MTX, methotrexate; VLB vinblastine) was studied by use of a computerized, kinetic chemosensitivity assay based on quantification of biomass by staining cells with crystal violet. For each compound the inhibition profile reflecting cytostatic, transient cytotoxic, or cytocidal drug effects as well as development of resistance was evaluated. The following order of activity was found: MTX >, VLB ≥ DX > cDDP ≥ 5-FU > JM-8. The test data of 5-FU, VLB, cDDP, and Tam on MXT+ as well as on MXT− were compared with those from studies on ER+ and ER− human breast cancer cell lines (MCF-7, ZR-75-1, T-47-D, and MDA-MB-231, respectively). They revealed comparable inhibition profiles and sensitivities of human and murine breast cancer cell lines, an indication that the results achieved in combined in vitro-/in vivo tests by use of the murine test models MXT+, MXT−, MXT-M-3, 2 MC, and MXT-M-3, 2(ovex) MC are relevant for therapy in humans.


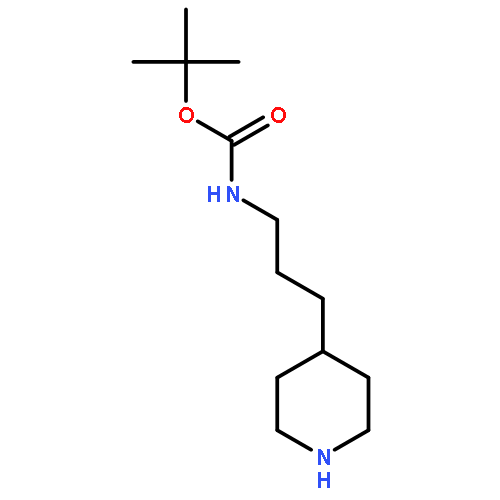













![Benzenamine, 4-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-2-iodo-](http://img.cochemist.com/ccimg/774300/774238-71-0.png)
![Benzenamine, 4-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-2-iodo-](http://img.cochemist.com/ccimg/774300/774238-71-0_b.png)
![4-[3-(1H-IMIDAZOL-4-YL)-PROPYL]-PIPERIDINE](http://img.cochemist.com/ccimg/768400/768358-61-8.png)
![4-[3-(1H-IMIDAZOL-4-YL)-PROPYL]-PIPERIDINE](http://img.cochemist.com/ccimg/768400/768358-61-8_b.png)