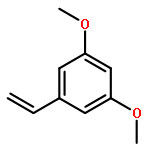
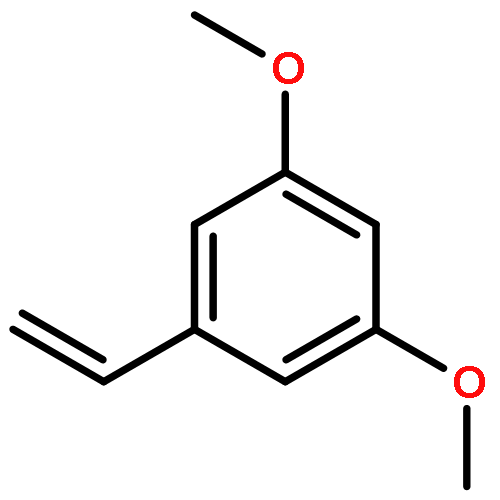
Co-reporter:Dongyin Chen;Fengguo Xu;Pu Zhang;Jie Deng;Hongbin Sun;Xiaoan Wen
Archiv der Pharmazie 2017 Volume 350(Issue 12) pp:
Publication Date(Web):2017/12/01
DOI:10.1002/ardp.201700178
A practical synthesis of α-amyrin (1), β-amyrin (2), and lupeol (3) was accomplished in total yields of 32, 42, and 40% starting from easily available ursolic acid (4), oleanolic acid (5), and betulin (6), respectively. Remarkably, these three natural pentacyclic triterpenes exhibited potential inhibitory activity against human oxidosqualene cyclase.
Co-reporter:Dongyin Chen, Xin Huang, Hongwen Zhou, Hanqiong Luo, Pengfei Wang, Yongzhi Chang, Xinyi He, Suiying Ni, Qingqing Shen, Guoshen Cao, Hongbin Sun, Xiaoan Wen, Jun Liu
European Journal of Medicinal Chemistry 2017 Volume 139(Volume 139) pp:
Publication Date(Web):20 October 2017
DOI:10.1016/j.ejmech.2017.08.012
•A series of pentacyclic triterpene 3β-ester derivatives were designed, synthesized and evaluated as a new class of CETP inhibitors.•The most potent compound 20 displayed an IC50 value of 2.3 μM against CETP in vitro.•Compound 20 effectively ameliorated plasma lipid levels of ap2-CETPTg mice and guinea pigs.•Compound 20 has a good pharmacokinetic profile.A series of pentacyclic triterpene 3β-ester derivatives were designed, synthesized and evaluated as a new class of cholesteryl ester transfer protein (CETP) inhibitors for the treatment of dyslipidemia. In vitro screening assay showed that 5 out of 30 compounds displayed moderate inhibiting human CETP activity with IC50s less than 10 μM. Among them, compound 20 (IC50 = 2.3 μM) had the most potent biological activity, and effectively ameliorated plasma lipid levels of human adipose tissue specific CETP transgenic (ap2-CETPTg) mice and guinea pigs. Additional safety evaluation (no blood pressure elevation in guinea pigs) and pharmacokinetics studies indicated that the potential druggability for compound 20 which is a promising lead for development of a new class of CETP inhibitors for the treatment of dyslipidemia.Download high-res image (154KB)Download full-size image
Co-reporter:Liying Zhang, Yingxia Zhang, Jizhe Dong, Jun Liu, Luyong Zhang, Hongbin Sun
Bioorganic & Medicinal Chemistry Letters 2012 Volume 22(Issue 2) pp:1036-1039
Publication Date(Web):15 January 2012
DOI:10.1016/j.bmcl.2011.11.123
To explore the molecular mechanisms of oleanolic acid, two novel photoaffinity probes were synthesized based on the structure–activity relationship reported previously. Their potency were evaluated in an enzyme inhibition assay against rabbit muscle glycogen phosphorylase a (RMGPa), a known target protein of oleanolic acid. The inhibitory activity of probe 2 was only about two-fold less potent than the mother compound oleanolic acid. The photoaffinity labeling experiments were also performed and two proteins were specifically tagged by probe 2. The results suggest that the synthesized probes could be used as powerful tools to isolate and identify the target proteins of oleanolic acid.











![Benzene, 1,2-dimethoxy-4-[(1E)-2-(4-nitrophenyl)ethenyl]-](http://img.cochemist.com/ccimg/51100/51042-54-7.png)
![Benzene, 1,2-dimethoxy-4-[(1E)-2-(4-nitrophenyl)ethenyl]-](http://img.cochemist.com/ccimg/51100/51042-54-7_b.png)