Co-reporter:Pengfei Sun, Min Jiang, Wei Wei, Yuanyuan Min, Wen Zhang, Wanhui Li, Daoshan Yang, and Hua Wang
The Journal of Organic Chemistry March 17, 2017 Volume 82(Issue 6) pp:2906-2906
Publication Date(Web):February 21, 2017
DOI:10.1021/acs.joc.6b02865
A convenient and efficient approach for the formation of nitrogen heterocycle-fused imidazo[1,2-a]pyridine and benzo[b]selenophenes has been developed through copper-catalyzed direct selenylation of readily available 2-(2-bromophenyl)imidazo[1,2-a]pyridines via regioselective cleavage of C(sp2)–Br and C(sp2)–H bonds using readily available selenium powder as the selenylating reagents under ligand- and base-free conditions in air. Preliminary mechanistic investigations indicated that radical species were involved in the present transformation.
Co-reporter:Pengfei Sun;Wei Wei;Linhong Jiang;Yuquan Wang;Tongxin Dai;Hua Wang
Organic Chemistry Frontiers 2017 vol. 4(Issue 7) pp:1367-1371
Publication Date(Web):2017/06/27
DOI:10.1039/C7QO00218A
A facile and eco-friendly protocol for the construction of C-4 sulfenylated pyrazoles via a radical pathway was established for the first time. The reaction worked smoothly under catalyst- and solvent-free conditions to afford a wide range of sulfenylated pyrazole derivatives in good to excellent yields. The thiyl free radical generated in situ, here, also served as a single electron transfer medium for the present transformation. This reaction provides a new strategy for the formation of C–S bonds.
Co-reporter:Pengfei Sun, Daoshan Yang, Wei Wei, Xuejun Sun, Wenhui Zhang, Hui Zhang, Yu Wang, Hua Wang
Tetrahedron 2017 Volume 73, Issue 15(Issue 15) pp:
Publication Date(Web):13 April 2017
DOI:10.1016/j.tet.2017.02.046
We describe herein a green and efficient MCRs protocol to synthesize C-4 sulfenylated pyrazoles by iodine-catalyzed cyclocondensation and direct CH bond sulphenylation reactions. Through this protocol, two new CN bonds and one CS bond are constructed simultaneously in a single step. This method provides a straightforward and sustainable way to construct valuable sulfenylated pyrazoles under metal- and solvent-free conditions.A facile access to C-4 sulfenylated pyrazoles via a domino multicomponent reaction has been realized under metal-free conditions.Download high-res image (285KB)Download full-size image
Co-reporter:Pengfei Sun;Wei Wei;Min Jiang;Zuli Wang;Liyan Zhang;Hui Zhang;Zhenzhen Zhang;Yu Wang;Hua Wang
Green Chemistry (1999-Present) 2017 vol. 19(Issue 20) pp:4785-4791
Publication Date(Web):2017/10/16
DOI:10.1039/C7GC01891F
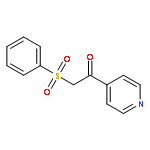
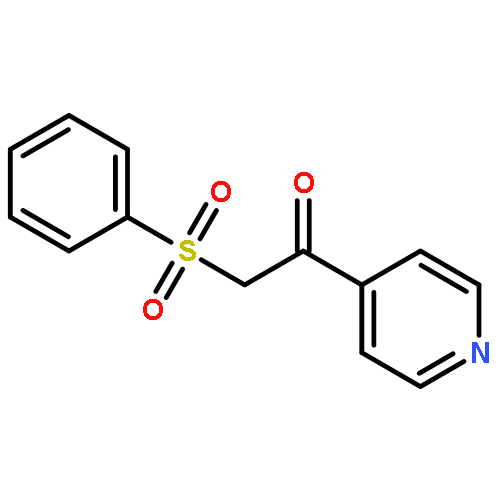
The visible light and Eosin B-catalyzed direct sulfenylation of sp2 C–H bonds with sulfinic acids through a photoredox process has been demonstrated at room temperature under transition metal-free conditions for the first time. Diverse heteroaryl sulfides were obtained in moderate to good yields. This is the first example of the sulfenylation of sp2 C–H bonds using arylsulfinic acids as odorless sulfur reagents under visible light-induced conditions. More interestingly, the reductive products could be obtained under visible light-induced oxidative conditions. This protocol demonstrates a new model for C–S bond formation, which serves as a novel approach toward the synthesis of heteroaryl sulfides.
Co-reporter:Daoshan Yang, Mingyang Sun, Wei Wei, Jin Li, Pengfei Sun, Qingyun Zhang, Laijin Tian and Hua Wang
RSC Advances 2016 vol. 6(Issue 76) pp:72361-72365
Publication Date(Web):25 Jul 2016
DOI:10.1039/C6RA16115D
An efficient copper-catalyzed decarboxylative coupling of cinnamic acids with N-fluorobenzenesulfonimide (NFSI) to give the corresponding substituted (E)-amination products stereospecifically is demonstrated. This new method is efficient and practical, and the corresponding products were obtained in moderate to good yields. The present protocol should broaden the scope of the decarboxylative cross-coupling reactions and provide a useful strategy for the construction of C–N bonds.
Co-reporter:Kelu Yan, Daoshan Yang, Wei Wei, Jing Zhao, Yuanyuan Shuai, Laijin Tian and Hua Wang
Organic & Biomolecular Chemistry 2015 vol. 13(Issue 26) pp:7323-7330
Publication Date(Web):26 May 2015
DOI:10.1039/C5OB00769K
A novel, efficient, and catalyst-free strategy has been initially developed for the construction of thioesters via the direct radical oxidative decarboxylation of α-keto acids with thiols, and the corresponding target products were obtained in moderate to good yields. It offers an alternative approach for the synthesis of useful diverse thioesters.
Co-reporter:Kelu Yan, Daoshan Yang, Wei Wei, Guoqing Li, Mingyang Sun, Qingyun Zhang, Laijin Tian and Hua Wang
RSC Advances 2015 vol. 5(Issue 121) pp:100102-100105
Publication Date(Web):17 Nov 2015
DOI:10.1039/C5RA17740E
A highly regioselective TBHP-mediated ring opening of imidazopyridines via cleavage of C–C and C–N bonds has been achieved for the first time to afford N-(pyridin-2-yl)benzamides. Preliminary mechanistic investigations revealed that the present metal-free transformation involved a radical pathway, and the oxygen atom incorporated in the end products might derive from TBHP.
Co-reporter:Ning Zhang, Daoshan Yang, Wei Wei, Li Yuan, Youjuan Cao and Hua Wang
RSC Advances 2015 vol. 5(Issue 46) pp:37013-37017
Publication Date(Web):14 Apr 2015
DOI:10.1039/C5RA02927A
Metal-free efficient iodine-mediated synthesis of vinyl sulfones utilizing aryl sulfinates and alkenes has been realized under mild conditions in water. Notably, sodium methanesulfinate was used in this transformation, affording the β-iodo sulfones in good yields. This simple, efficient and environmentally benign transformation provides an attractive approach to various vinyl sulfones or β-iodo sulfones.
Co-reporter:Kelu Yan, Daoshan Yang, Pengfei Sun, Wei Wei, Yao Liu, Guoqing Li, Shenglei Lu, Hua Wang
Tetrahedron Letters 2015 Volume 56(Issue 33) pp:4792-4795
Publication Date(Web):12 August 2015
DOI:10.1016/j.tetlet.2015.06.057
A direct thiolation of methoxybenzenes with various thiols has been developed. The protocol uses inexpensive reagents: catalytic iodine in the presence of DTBP. Importantly, no base or ligand was necessary. This method opens a new avenue to a variety of valuable thioethers that would be more difficult to access with traditional methods.A direct Thiolation of methoxybenzenes with various thiols has been developed. The protocol uses inexpensive reagents: catalytic iodine in the presence of DTBP. Importantly, no base or ligand was necessary. This method opens a new avenue to a variety of disulfides that would be more difficult to access with traditional methods.
Co-reporter:Ning Zhang, Daoshan Yang, Wei Wei, Li Yuan, Fafa Nie, Laijin Tian, and Hua Wang
The Journal of Organic Chemistry 2015 Volume 80(Issue 6) pp:3258-3263
Publication Date(Web):February 20, 2015
DOI:10.1021/jo502642n
A silver-catalyzed double-decarboxylative protocol has been proposed for the construction of chalcone derivatives via cascade coupling of substituted α-keto acids with cinnamic acids under the mild aqueous conditions. The developed method for constructing C–C bonds via double-decarboxylative reactions is efficient, practical, and environmentally benign by using the readily available starting materials. It should provide a promising synthesis candidate for the formation of diverse and useful chalcone derivatives in the fields of synthetic and pharmaceutical chemistry.
Co-reporter:Kelu Yan, Daoshan Yang, Wei Wei, Fen Wang, Yuanyuan Shuai, Qiannan Li, and Hua Wang
The Journal of Organic Chemistry 2015 Volume 80(Issue 3) pp:1550-1556
Publication Date(Web):January 6, 2015
DOI:10.1021/jo502474z
A novel and convenient silver-mediated radical cyclization method for the synthesis of coumarin derivatives via the direct difunctionalization of alkynoates with α-keto acids through double C–C bond formation under mild conditions has been developed. This new method is highly efficient and practical, and the starting materials are readily prepared. The present method should provide a useful strategy for the construction of coumarin motifs.
Co-reporter:Xiao Zhu, Daoshan Yang, Wei Wei, Min Jiang, Lulu Li, Xiangbing Zhu, Jinmao You and Hua Wang
RSC Advances 2014 vol. 4(Issue 110) pp:64930-64935
Publication Date(Web):20 Nov 2014
DOI:10.1039/C4RA14152K
A novel, effective and sustainable strategy for the synthesis of aldehydes has been developed using inexpensive, readily available, oxygen-stable and recyclable CuFe2O4 nanoparticles as the catalyst. The corresponding substituted aldehydes were obtained in moderate to good yields by aerobic oxidation of aromatic alcohols in water under dioxygen atmosphere. Importantly, a ligand or a base was not necessary. The catalyst was completely recoverable with an external magnet and could be reused six times without significant loss of catalytic activity.
Co-reporter:Kelu Yan, Daoshan Yang, Wei Wei, Jing Zhao, Yuanyuan Shuai, Laijin Tian and Hua Wang
Organic & Biomolecular Chemistry 2015 - vol. 13(Issue 26) pp:NaN7330-7330
Publication Date(Web):2015/05/26
DOI:10.1039/C5OB00769K
A novel, efficient, and catalyst-free strategy has been initially developed for the construction of thioesters via the direct radical oxidative decarboxylation of α-keto acids with thiols, and the corresponding target products were obtained in moderate to good yields. It offers an alternative approach for the synthesis of useful diverse thioesters.
Co-reporter:Pengfei Sun, Daoshan Yang, Wei Wei, Linhong Jiang, Yuquan Wang, Tongxin Dai and Hua Wang
Inorganic Chemistry Frontiers 2017 - vol. 4(Issue 7) pp:NaN1371-1371
Publication Date(Web):2017/04/17
DOI:10.1039/C7QO00218A
A facile and eco-friendly protocol for the construction of C-4 sulfenylated pyrazoles via a radical pathway was established for the first time. The reaction worked smoothly under catalyst- and solvent-free conditions to afford a wide range of sulfenylated pyrazole derivatives in good to excellent yields. The thiyl free radical generated in situ, here, also served as a single electron transfer medium for the present transformation. This reaction provides a new strategy for the formation of C–S bonds.

![3-Chloro-6-(trifluoromethyl)benzo[b]thiophene-2-carboxylic acid](http://img.cochemist.com/ccimg/923900/923849-73-4.png)
![3-Chloro-6-(trifluoromethyl)benzo[b]thiophene-2-carboxylic acid](http://img.cochemist.com/ccimg/923900/923849-73-4_b.png)
![Benzamide, N-[2-bromo-5-(trifluoromethyl)phenyl]-](http://img.cochemist.com/ccimg/880900/880883-58-9.png)
![Benzamide, N-[2-bromo-5-(trifluoromethyl)phenyl]-](http://img.cochemist.com/ccimg/880900/880883-58-9_b.png)
![8-Methyl-2-phenylimidazo[1,2-a]pyridin-3-amine](http://img.cochemist.com/ccimg/850100/850020-93-8.png)
![8-Methyl-2-phenylimidazo[1,2-a]pyridin-3-amine](http://img.cochemist.com/ccimg/850100/850020-93-8_b.png)