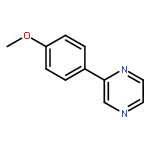
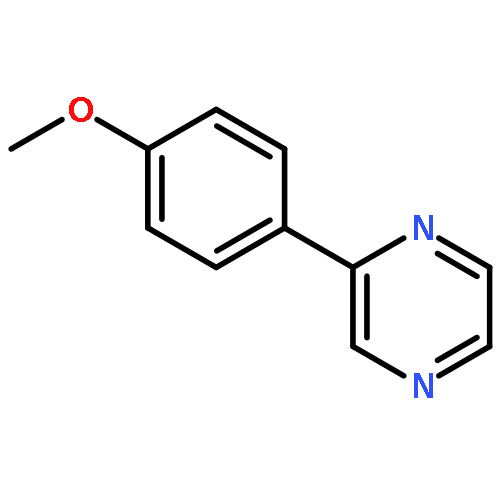
Co-reporter:Xue Jiang, Weijun Tang, Dong Xue, Jianliang Xiao, and Chao Wang
ACS Catalysis March 3, 2017 Volume 7(Issue 3) pp:1831-1831
Publication Date(Web):January 31, 2017
DOI:10.1021/acscatal.6b03667
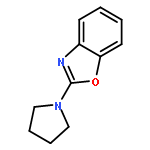
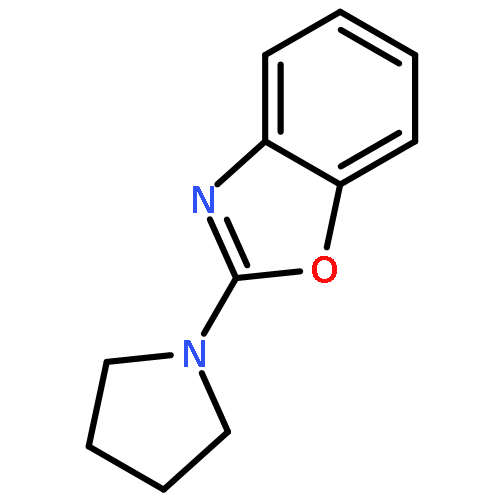
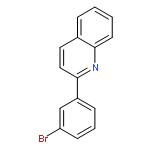
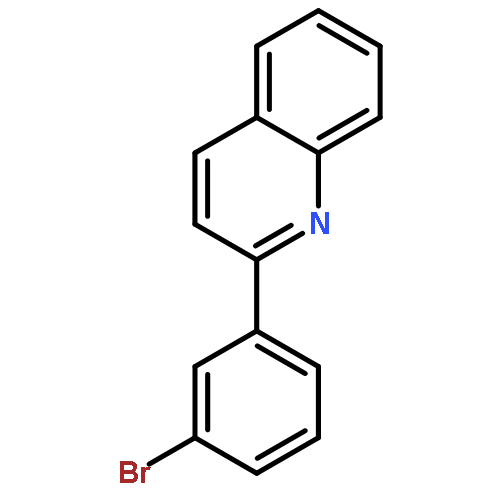
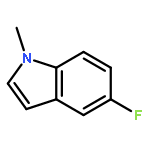
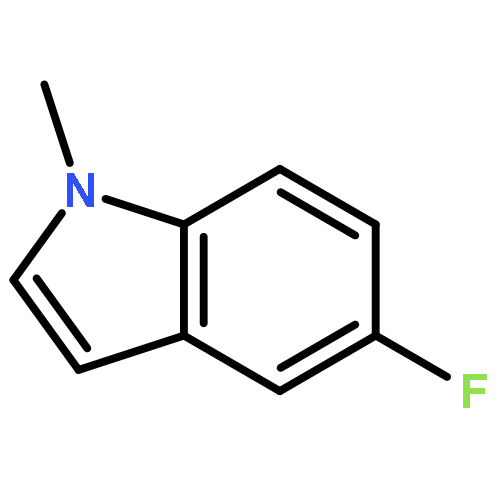
The dehydrogenative coupling of indolines with alcohols catalyzed by an iridium complex has been achieved to afford both N- and C3-alkylated indoles selectively, by simply changing the addition time of a base additive. The iridacycle catalyst plays multiple roles in these reactions, which dehydrogenates both amines and alcohols and catalyzes the coupling reactions. Mechanistic studies reveal that a borrowing hydrogen-dehydrogenation process and a dehydrogenation-borrowing hydrogen process are involved in N-alkylation and C3-alkylation reactions, respectively. The C3-alkylation reaction involves the direct coupling of two sp3 carbon centers.Keywords: alcohol; cross coupling; dehydrogenation; indole; iridium;
Co-reporter:Yuxia Liu;Dong Xue;Chaoqun Li;Jianliang Xiao
Catalysis Science & Technology (2011-Present) 2017 vol. 7(Issue 23) pp:5510-5514
Publication Date(Web):2017/11/27
DOI:10.1039/C7CY01757J
Terminal alkenes were selectively cleaved into ketones and aldehydes catalyzed by a binuclear copper catalyst bearing a simple salicylate ligand with O2 as the oxidant. The reaction was carried out under an atmosphere of O2 (balloon) with 0.5 mol% of catalyst and could be performed on a gram scale, providing a convenient and practical method for the cleavage of terminal alkenes into carbonyl compounds.
Co-reporter:Xuewei Wang, Chao Wang, Yuxuan Liu and Jianliang Xiao
Green Chemistry 2016 vol. 18(Issue 17) pp:4605-4610
Publication Date(Web):27 Jun 2016
DOI:10.1039/C6GC01272H
A water-soluble binuclear rhodium(II) complex was found to be an efficient catalyst for both acceptorless dehydrogenation and aerobic oxidation of alcohols under air to produce carboxylic acids or ketones in water. The catalyst is highly efficient with substrate/catalyst ratios up to 5 × 103 being feasible, and could be recycled 19 times without significant loss of activity.
Co-reporter:Junjie Cheng, Meijuan Zhu, Chao Wang, Junjun Li, Xue Jiang, Yawen Wei, Weijun Tang, Dong Xue and Jianliang Xiao
Chemical Science 2016 vol. 7(Issue 7) pp:4428-4434
Publication Date(Web):29 Mar 2016
DOI:10.1039/C6SC00145A
Dehydrogenative cross-coupling of aldehydes with alcohols as well as dehydrogentive cross-coupling of primary alcohols to produce esters have been developed using a Rh-terpyridine catalyst. The catalyst demonstrates broad substrate scope and good functional group tolerance, affording esters highly selectively. The high chemoselectivity of the catalyst stems from its preference for dehydrogenation of benzylic alcohols over aliphatic ones. Preliminary mechanistic studies suggest that the active catalyst is a dimeric Rh(II) species, operating via a mechanism involving metal–base–metal cooperativity.
Co-reporter:Bohua Wu, Chao Wang, Ying Cui, Liqiu Mao and Shanxin Xiong
RSC Advances 2015 vol. 5(Issue 22) pp:16986-16992
Publication Date(Web):22 Jan 2015
DOI:10.1039/C4RA14659J
Taking the direct Friedel–Crafts reaction between pristine carbon nanotubes (CNT) and maleic anhydride, we have developed a facile strategy for the synthesis of carboxylated-carbon nanotubes (CNT-C). The covalently grafted carboxyl groups on the CNT-C surface not only significantly improves its hydrophilicity, but also effectively anchors and stabilizes the PtRu nanoparticles. Transmission electron microscopy images reveal that PtRu nanoparticles with an average size of 3.3 nm are uniformly dispersed on the CNT-C surface. Electrochemical results demonstrate the PtRu/CNT-C nanohybrids obtained have a higher electrochemical surface area, electrocatalytic activity and better stability towards methanol oxidation when compared to PtRu nanoparticles supported on acid oxidized-CNT. This provides a facile approach to synthesize noble metal nanoparticles/CNT electrocatalysts for high performance energy conversion devices in the future.
Co-reporter:Meijuan Zhu, Chao Wang, Weijun Tang, Jianliang Xiao
Tetrahedron Letters 2015 Volume 56(Issue 48) pp:6758-6761
Publication Date(Web):2 December 2015
DOI:10.1016/j.tetlet.2015.10.062
A method for the synthesis of quinolines from cheap and readily available 2-nitrobenzyl alcohol without a transition-metal catalyst in water has been developed, providing a convenient method for accessing quinolines. The reaction features an intramolecular redox process, which generates the key intermediate leading to product formation.
Co-reporter:Qingzhu Zou;Dr. Chao Wang;Jen Smith;Dr. Dong Xue;Dr. Jianliang Xiao
Chemistry - A European Journal 2015 Volume 21( Issue 27) pp:9656-9661
Publication Date(Web):
DOI:10.1002/chem.201501109
Abstract
An efficient catalytic system for the alkylation of amines with either alcohols or amines under mild conditions has been developed, using cyclometallated iridium complexes as catalysts. The method has broad substrate scope, allowing for the synthesis of a diverse range of secondary and tertiary amines with good to excellent yields. By controlling the ratio of substrates, both mono- and bis-alkylated amines can be obtained with high selectivity. In particular, methanol can be used as the alkylating reagent, affording N-methylated products selectively. A strong solvent effect is observed for the reaction.
Co-reporter:Yawen Wei, Chao Wang, Xue Jiang, Dong Xue, Zhao-Tie Liu and Jianliang Xiao
Green Chemistry 2014 vol. 16(Issue 3) pp:1093-1096
Publication Date(Web):11 Nov 2013
DOI:10.1039/C3GC42125B
Levulinic acid (LA) is transformed into pyrrolidinones by formic acid in DMSO without a catalyst. Mechanistic studies suggest the involvement of an iminium intermediate and a rate-limiting hydride transfer step.
Co-reporter:Xue Jiang;Dr. Chao Wang;Yawen Wei;Dr. Dong Xue;Dr. Zhaotie Liu;Dr. Jianliang Xiao
Chemistry - A European Journal 2014 Volume 20( Issue 1) pp:58-63
Publication Date(Web):
DOI:10.1002/chem.201303802
Abstract
DMSO methylates a broad range of amines in the presence of formic acid, providing a novel, green and practical method for amine methylation. The protocol also allows the one-pot transformation of aromatic nitro compounds into dimethylated amines in the presence of a simple iron catalyst.
Co-reporter:Jia Li, Chao Wang, Dong Xue, Yawen Wei and Jianliang Xiao
Green Chemistry 2013 vol. 15(Issue 10) pp:2685-2689
Publication Date(Web):02 Aug 2013
DOI:10.1039/C3GC41133H
Alkynes are converted into alcohols and amines through a formic acid-participated alkyne-to-ketone transformation and transfer hydrogenation process. The reaction proceeds well under aqueous conditions, furnishing chiral alcohols directly from alkynes for the first time.
Co-reporter:Yawen Wei, Chao Wang, Xue Jiang, Dong Xue, Jia Li and Jianliang Xiao
Chemical Communications 2013 vol. 49(Issue 47) pp:5408-5410
Publication Date(Web):23 Apr 2013
DOI:10.1039/C3CC41661E
Levulinic acid (LA) is transformed into pyrrolidinones via iridium-catalysed reductive amination using formic acid as the hydrogen source under aqueous conditions. The catalytic system is the most active and performs under the mildest conditions ever reported for the reductive amination of LA.
Co-reporter:Qian Lei;Yawen Wei;Dinesh Talwar; Chao Wang; Dong Xue; Jianliang Xiao
Chemistry - A European Journal 2013 Volume 19( Issue 12) pp:4021-4029
Publication Date(Web):
DOI:10.1002/chem.201204194
Abstract
Reductive amination of various ketones and aldehydes by transfer hydrogenation under aqueous conditions has been developed, by using cyclometallated iridium complexes as catalysts and formate as hydrogen source. The pH value of the solution is shown to be critical for a high catalytic chemoselectivity and activity, with the best pH value being 4.8. In comparison with that in organic solvents, the reductive amination in an aqueous phase is faster, and the molar ratio of the substrate to the catalyst (S/C) can be set as high as 1×105, the highest S/C value ever reported in reductive amination reactions. The catalyst is easy to access and the reaction is operationally simple, allowing a wide range of ketones and aldehydes to react with various amines in high yields. The protocol provides a practical and environmental friendly new method for the synthesis of amine compounds.
Co-reporter:Yawen Wei, Chao Wang, Xue Jiang, Dong Xue, Jia Li and Jianliang Xiao
Chemical Communications 2013 - vol. 49(Issue 47) pp:NaN5410-5410
Publication Date(Web):2013/04/23
DOI:10.1039/C3CC41661E
Levulinic acid (LA) is transformed into pyrrolidinones via iridium-catalysed reductive amination using formic acid as the hydrogen source under aqueous conditions. The catalytic system is the most active and performs under the mildest conditions ever reported for the reductive amination of LA.
Co-reporter:Junjie Cheng, Meijuan Zhu, Chao Wang, Junjun Li, Xue Jiang, Yawen Wei, Weijun Tang, Dong Xue and Jianliang Xiao
Chemical Science (2010-Present) 2016 - vol. 7(Issue 7) pp:NaN4434-4434
Publication Date(Web):2016/03/29
DOI:10.1039/C6SC00145A
Dehydrogenative cross-coupling of aldehydes with alcohols as well as dehydrogentive cross-coupling of primary alcohols to produce esters have been developed using a Rh-terpyridine catalyst. The catalyst demonstrates broad substrate scope and good functional group tolerance, affording esters highly selectively. The high chemoselectivity of the catalyst stems from its preference for dehydrogenation of benzylic alcohols over aliphatic ones. Preliminary mechanistic studies suggest that the active catalyst is a dimeric Rh(II) species, operating via a mechanism involving metal–base–metal cooperativity.

![1H-Indole, 3-[(4-nitrophenyl)methyl]-](http://img.cochemist.com/ccimg/114100/114092-96-5.png)
![1H-Indole, 3-[(4-nitrophenyl)methyl]-](http://img.cochemist.com/ccimg/114100/114092-96-5_b.png)