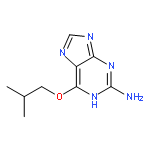
Co-reporter:Andrey V. Zaytsev, James E. Pickles, Suzannah J. Harnor, Alistair P. Henderson, Mohammed Alyasiri, Paul G. Waddell, Celine Cano, Roger J. Griffin and Bernard T. Golding
RSC Advances 2016 vol. 6(Issue 59) pp:53955-53957
Publication Date(Web):27 May 2016
DOI:10.1039/C6RA08737J
Concise and practical syntheses of 8-oxa-3-aza-bicyclo[3.2.1]octane and 9-oxa-3-aza-bicyclo[3.3.1]nonane are described starting from furan-2,5-dicarboxylic acid and 4H-pyran-2,6-dicarboxylic acid, respectively, and using a solvent-free step for a key cyclisation.
Co-reporter:Elizabeth Anscombe, Elisa Meschini, Regina Mora-Vidal, Mathew P. Martin, David Staunton, Matthis Geitmann, U. Helena Danielson, Will A. Stanley, Lan Z. Wang, Tristan Reuillon, Bernard T. Golding, Celine Cano, David R. Newell, Martin E.M. Noble, Stephen R. Wedge, Jane A. Endicott, Roger J. Griffin
Chemistry & Biology 2015 Volume 22(Issue 9) pp:1159-1164
Publication Date(Web):17 September 2015
DOI:10.1016/j.chembiol.2015.07.018
•NU6300 is the first example of a covalent CDK2 inhibitor•A CDK2/cyclin A/NU6300 co-crystal structure reveals the inhibitor binding mode•NU6300 is active in cellsIrreversible inhibitors that modify cysteine or lysine residues within a protein kinase ATP binding site offer, through their distinctive mode of action, an alternative to ATP-competitive agents. 4-((6-(Cyclohexylmethoxy)-9H-purin-2-yl)amino)benzenesulfonamide (NU6102) is a potent and selective ATP-competitive inhibitor of CDK2 in which the sulfonamide moiety is positioned close to a pair of lysine residues. Guided by the CDK2/NU6102 structure, we designed 6-(cyclohexylmethoxy)-N-(4-(vinylsulfonyl)phenyl)-9H-purin-2-amine (NU6300), which binds covalently to CDK2 as shown by a co-complex crystal structure. Acute incubation with NU6300 produced a durable inhibition of Rb phosphorylation in SKUT-1B cells, consistent with it acting as an irreversible CDK2 inhibitor. NU6300 is the first covalent CDK2 inhibitor to be described, and illustrates the potential of vinyl sulfones for the design of more potent and selective compounds.Figure optionsDownload full-size imageDownload high-quality image (233 K)Download as PowerPoint slide
Co-reporter:Honorine Lebraud, Christopher R. Coxon, Victoria S. Archard, Carlo M. Bawn, Benoit Carbain, Christopher J. Matheson, David M. Turner, Celine Cano, Roger J. Griffin, Ian R. Hardcastle, Ulrich Baisch, Ross W. Harrington and Bernard T. Golding
Organic & Biomolecular Chemistry 2014 vol. 12(Issue 1) pp:141-148
Publication Date(Web):29 Oct 2013
DOI:10.1039/C3OB41806E
Recent studies have shown that irreversible inhibition of Nek2 kinase [(Never in mitosis gene a)-related kinase 2], overexpression of which is observed in several cancers, can be achieved using Michael acceptors containing an ethynyl group, which target the enzyme's cysteine 22 residue lying near the catalytic site. The model studies described herein demonstrate an analogous capture of the ethynyl moiety in a series of ethynyl-heterocycles (e.g. 6-ethynyl-N-phenyl-9H-purin-2-amine) by N-acetylcysteine methyl ester in the presence of 1,4-diazabicyclo[2.2.2]octane in either dimethyl sulfoxide or N,N-dimethylformamide. Kinetic studies showed a 50-fold range in reactivity with 7-ethynyl-N-phenyl-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-amine being the most reactive compound, whereas 4-ethynyl-N-phenyl-7H-pyrrolo[2,3-d]pyrimidin-2-amine was the least reactive. Studies of the isomeric compounds, 2-(3-((6-ethynyl-7-methyl-7H-purin-2-yl)amino)phenyl)acetamide and 2-(3-((6-ethynyl-9-methyl-9H-purin-2-yl)amino)phenyl)acetamide, revealed the N7-methyl isomer to be 5-fold more reactive than the 9-methyl isomer, which is ascribed to a buttressing effect in the N7-methyl compound. Comparison of the crystal structures of these isomers showed that the ethynyl group is significantly displaced away from the methyl group exclusively in the N7-methyl isomer with an sp2 bond angle of 124°, whereas the corresponding angle in the N9-methyl isomer was the expected 120°. The results of this study indicate heterocyclic scaffolds that are likely to be more promising for inhibition of Nek2 and other kinases containing a reactive cysteine.
Co-reporter: Bernard T. Golding; Wolfgang Buckel
Angewandte Chemie International Edition 2014 Volume 53( Issue 14) pp:
Publication Date(Web):
DOI:10.1002/anie.201401077
Co-reporter: Bernard T. Golding; Wolfgang Buckel
Angewandte Chemie 2014 Volume 126( Issue 14) pp:
Publication Date(Web):
DOI:10.1002/ange.201401077
Co-reporter:Honorine Lebraud, Christopher R. Coxon, Victoria S. Archard, Carlo M. Bawn, Benoit Carbain, Christopher J. Matheson, David M. Turner, Celine Cano, Roger J. Griffin, Ian R. Hardcastle, Ulrich Baisch, Ross W. Harrington and Bernard T. Golding
Organic & Biomolecular Chemistry 2014 - vol. 12(Issue 1) pp:NaN148-148
Publication Date(Web):2013/10/29
DOI:10.1039/C3OB41806E
Recent studies have shown that irreversible inhibition of Nek2 kinase [(Never in mitosis gene a)-related kinase 2], overexpression of which is observed in several cancers, can be achieved using Michael acceptors containing an ethynyl group, which target the enzyme's cysteine 22 residue lying near the catalytic site. The model studies described herein demonstrate an analogous capture of the ethynyl moiety in a series of ethynyl-heterocycles (e.g. 6-ethynyl-N-phenyl-9H-purin-2-amine) by N-acetylcysteine methyl ester in the presence of 1,4-diazabicyclo[2.2.2]octane in either dimethyl sulfoxide or N,N-dimethylformamide. Kinetic studies showed a 50-fold range in reactivity with 7-ethynyl-N-phenyl-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-amine being the most reactive compound, whereas 4-ethynyl-N-phenyl-7H-pyrrolo[2,3-d]pyrimidin-2-amine was the least reactive. Studies of the isomeric compounds, 2-(3-((6-ethynyl-7-methyl-7H-purin-2-yl)amino)phenyl)acetamide and 2-(3-((6-ethynyl-9-methyl-9H-purin-2-yl)amino)phenyl)acetamide, revealed the N7-methyl isomer to be 5-fold more reactive than the 9-methyl isomer, which is ascribed to a buttressing effect in the N7-methyl compound. Comparison of the crystal structures of these isomers showed that the ethynyl group is significantly displaced away from the methyl group exclusively in the N7-methyl isomer with an sp2 bond angle of 124°, whereas the corresponding angle in the N9-methyl isomer was the expected 120°. The results of this study indicate heterocyclic scaffolds that are likely to be more promising for inhibition of Nek2 and other kinases containing a reactive cysteine.


![Benzoic acid, 4-[[2-(trimethylsilyl)ethoxy]methoxy]-, ethyl ester](http://img.cochemist.com/ccimg/849800/849723-54-2.png)
![Benzoic acid, 4-[[2-(trimethylsilyl)ethoxy]methoxy]-, ethyl ester](http://img.cochemist.com/ccimg/849800/849723-54-2_b.png)










![4H-Thiopyran-4-one, 2-(4'-hydroxy[1,1'-biphenyl]-3-yl)-6-(4-morpholinyl)-](http://img.cochemist.com/ccimg/500200/500169-59-5.png)
![4H-Thiopyran-4-one, 2-(4'-hydroxy[1,1'-biphenyl]-3-yl)-6-(4-morpholinyl)-](http://img.cochemist.com/ccimg/500200/500169-59-5_b.png)