Co-reporter:Rohit Singh Chauhan, Neelam Shivran, Alexandra M.Z. Slawin, J. Derek Woollins
Journal of Organometallic Chemistry 2017 Volume 830(Volume 830) pp:
Publication Date(Web):15 February 2017
DOI:10.1016/j.jorganchem.2016.12.013
•Reactions of [Pt(SeC5H4N)2(dppm)] with d10 metal system resulted [PtX2(dppm)].•Reactions of [Pt(SeC5H4N)2(dppp)] with d10 metal system yielded an additive product.•Interaction between Pt-d10depends on the size of metal.Reactions of [Pt(SeC5H4N)2(P∩P)] with M(OAc)2.2H2O/MX2 (P∩P = dppm, dppp; M = Zn, Cd, Hg, Cu; X = Cl) resulted various products depend upon the nature of the phosphine. In case of dppm, reaction between [Pt(SeC5H4N)2(dppm)] and M(OAc)2.2H2O/MX2 (M = Zn, Cd, Hg) afforded an exchange product of composition [PtCl2(dppm)]. Similarly, the reaction with ‘dppp’ analog yielded an adduct [{Pt(SeC5H4N)2(dppp)}MX2] (M = Cd, Hg, Cu; X = Cl, OAc). Whereas, reaction with Zn(OAc)2, on extraction with dichloromethane resulted the product with the loss of one selenium atom of composition [{Pt(C5H4N)(SeC5H4N)(dppp)}ZnCl2]. These complexes were characterized by elemental analyses and NMR (1H, 31P, 77Se, 195Pt) spectroscopy. The molecular structure of [{Pt(C5H4N)(SeC5H4N)(dppp)}ZnCl2] was established by single crystal X-ray diffraction analyses.Download high-res image (176KB)Download full-size image
Co-reporter:Susan E. Henkelis, Laura J. McCormick, David B. Cordes, Alexandra M.Z. Slawin, Russell E. Morris
Inorganic Chemistry Communications 2016 Volume 65() pp:21-23
Publication Date(Web):March 2016
DOI:10.1016/j.inoche.2016.01.007
•The first example of a non-polymeric Mg-H2dhtp complex is reported.•Obtained by one-pot reflux at lower pH than leads to a known MOF, CPO-27-Mg•Hydrogen bonding interactions construct a complex 3D network.A mononuclear complex of composition Mg(H2dhtp)(H2O)5·H2O has been prepared and characterised crystallographically.A new mononuclear Mg-dhtp compound with ligand 2,5-dihydroxyterephathilic acid (dhtp) was prepared and its crystal structure was determined.
Co-reporter:Vladimiros A. Nikolakis, Vassiliki Exarchou, Tamás Jakusch, J. Derek Woolins, Alexandra M. Z. Slawin, Tamás Kiss and Themistoklis A. Kabanos
Dalton Transactions 2010 vol. 39(Issue 38) pp:9032-9038
Publication Date(Web):24 Aug 2010
DOI:10.1039/C0DT00574F
The treatment of the trichloro-1,3,5-triazine with N-methylhydroxylamine hydrochloride results in the replacement of the three chlorine atoms of the triazine ring with the function –N(OH)CH3 yielding the symmetrical tris-(hydroxyamino)triazine ligand H3trihyat. Reaction of the ligand H3trihyat with NaVVO3 in aqueous solution followed by addition of Ph4PCl gave the mononuclear vanadium(V) compound Ph4P[VVO2(Htrihyat)] (1). The structure of compound 1 was determined by X-ray crystallography and indicates that this compound has a distorted square-pyramidal arrangement around vanadium. The ligand Htrihyat2− is bonded to vanadium atom in a tridentate fashion at the triazine ring nitrogen atom and the two deprotonated hydroxylamido oxygen atoms. The high electron density of the triazine ring nitrogen atoms, which results from the resonative contribution of electrons of exocyclic nitrogen atoms, leads to a very strong V–N bond. The cis-[VVO2(Htrihyat)]− species exhibits high hydrolytic stability in aqueous solution over a wide pH range, 2.5–11.5, as was evidenced by potentiometry.
Co-reporter:Vladimiros A. Nikolakis, Vassiliki Exarchou, Tamás Jakusch, J. Derek Woolins, Alexandra M. Z. Slawin, Tamás Kiss and Themistoklis A. Kabanos
Dalton Transactions 2010 - vol. 39(Issue 38) pp:NaN9038-9038
Publication Date(Web):2010/08/24
DOI:10.1039/C0DT00574F
The treatment of the trichloro-1,3,5-triazine with N-methylhydroxylamine hydrochloride results in the replacement of the three chlorine atoms of the triazine ring with the function –N(OH)CH3 yielding the symmetrical tris-(hydroxyamino)triazine ligand H3trihyat. Reaction of the ligand H3trihyat with NaVVO3 in aqueous solution followed by addition of Ph4PCl gave the mononuclear vanadium(V) compound Ph4P[VVO2(Htrihyat)] (1). The structure of compound 1 was determined by X-ray crystallography and indicates that this compound has a distorted square-pyramidal arrangement around vanadium. The ligand Htrihyat2− is bonded to vanadium atom in a tridentate fashion at the triazine ring nitrogen atom and the two deprotonated hydroxylamido oxygen atoms. The high electron density of the triazine ring nitrogen atoms, which results from the resonative contribution of electrons of exocyclic nitrogen atoms, leads to a very strong V–N bond. The cis-[VVO2(Htrihyat)]− species exhibits high hydrolytic stability in aqueous solution over a wide pH range, 2.5–11.5, as was evidenced by potentiometry.


![Naphtho[1,8-cd]-1,2-dithiole, 1,1,2,2-tetraoxide](http://img.cochemist.com/ccimg/62700/62609-77-2.png)
![Naphtho[1,8-cd]-1,2-dithiole, 1,1,2,2-tetraoxide](http://img.cochemist.com/ccimg/62700/62609-77-2_b.png)


![Phosphine, diphenyl[8-(phenylthio)-1-naphthalenyl]-](/data/chemimg/3726200/1198294-18-6.png)
![Phosphine, diphenyl[8-(phenylthio)-1-naphthalenyl]-](/data/chemimg/3726200/1198294-18-6_b.png)



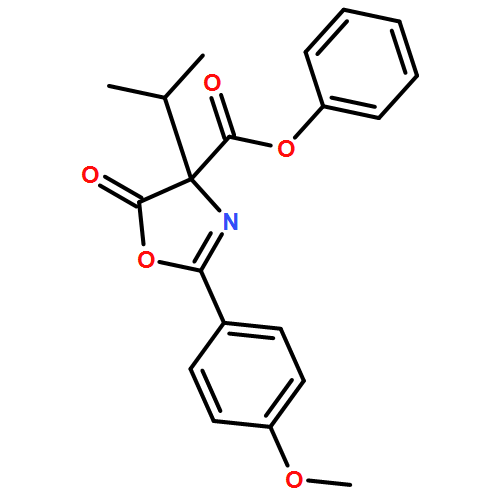
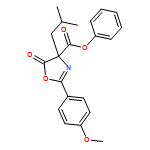
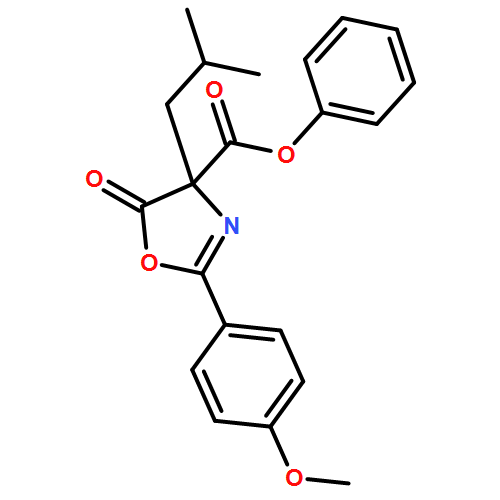
![4-Oxazolecarboxylic acid, 4,5-dihydro-2-(4-methoxyphenyl)-4-[2-(methylthio)ethyl]-5-oxo-, phenyl ester](/data/chemimg/3721700/1017848-92-8.png)
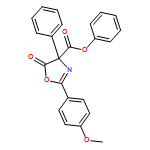
![4-Oxazolecarboxylic acid, 4,5-dihydro-2-(4-methoxyphenyl)-4-[2-(methylthio)ethyl]-5-oxo-, phenyl ester](/data/chemimg/3721700/1017848-92-8_b.png)