Co-reporter:Jun Jiang, Huaxu Zou, Qizhi Dong, Ruijia Wang, Linghui Lu, Yonggang Zhu, and Weimin He
The Journal of Organic Chemistry 2016 Volume 81(Issue 1) pp:51-56
Publication Date(Web):December 16, 2015
DOI:10.1021/acs.joc.5b02093
An interesting base-promoted protocol for the synthesis of 2-keto(hetero)aryl benzox(thi)azoles has been developed. Starting from commercially available 2-amino(thio)phenols and α,α-dihaloketones, moderate to good yields of the corresponding heterocycles can be achieved. Notably, only EtNH2 was required as the promoter here, and the reaction can be easily performed on a large scale.
Co-reporter:Jiannan Xiang, Niannian Yi, Ruijia Wang, Linghui Lu, Huaxu Zou, Yuan Pan, Weimin He
Tetrahedron 2015 Volume 71(Issue 4) pp:694-699
Publication Date(Web):28 January 2015
DOI:10.1016/j.tet.2014.12.001
β-Ketophosphonates were prepared via AgNO3-catalyzed hydration of alkynylphosphonates with a dramatic rate-enhancement effect of methanol. This benign aqueous-methanol method catalyzed by a low-cost catalyst has simple, atom-economical procedure, and was used effectively with a wide range of substrates.
Co-reporter:Niannian Yi, Ruijia Wang, Huaxu Zou, Weibao He, Wenqiang Fu, and Weimin He
The Journal of Organic Chemistry 2015 Volume 80(Issue 10) pp:5023-5029
Publication Date(Web):April 19, 2015
DOI:10.1021/acs.joc.5b00408
A copper/iron-catalyzed oxyphosphorylation of alkynes with H-phosphonates through a radical process was developed. The present protocol provides an attractive approach to β-ketophosphonates in moderate to good yields, with the advantages of readily available substrate, high functional group tolerance and operation simplicity.
Co-reporter:Longyong Xie;Rui Yuan;Ruijia Wang;Zhihong Peng;Jiannan Xiang
European Journal of Organic Chemistry 2014 Volume 2014( Issue 13) pp:2668-2671
Publication Date(Web):
DOI:10.1002/ejoc.201400066
Abstract
A general, efficient, and highly regioselective protocol with the use of a gold(I) complex catalytic system for the transformation of alkynylphosphonates into the corresponding β-ketophosphonates has been successfully developed. This method produces a variety of β-ketophosphonates with the advantages of mild reaction conditions, high functional-group tolerance, and excellent yields.
Co-reporter:Jiannan Xiang, Rui Yuan, Ruijia Wang, Niannian Yi, Linghui Lu, Huaxu Zou, and Weimin He
The Journal of Organic Chemistry 2014 Volume 79(Issue 23) pp:11378-11382
Publication Date(Web):November 19, 2014
DOI:10.1021/jo501776b
The highly stereoselective bromination of alkynes has been realized by using copper(II) bromide as both the reacting partner and the catalyst, offering a generally efficient synthesis of (E)-dibromoalkenes. The reaction conditions are exceptionally mild, and a wide range of functional groups are well tolerated.
Co-reporter:Chao Wu, Zhi-Wu Liang, Ying-Ying Xu, Wei-Min He, Jian-Nan Xiang
Chinese Chemical Letters 2013 Volume 24(Issue 12) pp:1064-1066
Publication Date(Web):December 2013
DOI:10.1016/j.cclet.2013.06.026
Nine 5-aryl-2-methyloxazole derivatives were synthesized via gold-catalyzed alkyne oxidation. All of the compounds have been screened for their antiproliferative activities against MCF-7 cell (human breast carcinoma), A549 cell (human lung carcinoma) and Hela cell (human cervical carcinoma) lines in vitro. The results revealed that compounds 1b, 1c and 1d exhibited strong inhibitory activities against the MCF-7 cell lines (with IC50 values of 4.6, 9.7 and 2.2 μmol/L, respectively).Nine 5-aryl-2-methyloxazole derivatives were synthesized via gold-catalyzed alkyne oxidation, and compounds 1b, 1c and 1d exhibited the strong inhibitory activities against the MCF-7 cell lines.
Co-reporter:Longyong Xie, Yundong Wu, Weiguo Yi, Lei Zhu, Jiannan Xiang, and Weimin He
The Journal of Organic Chemistry 2013 Volume 78(Issue 18) pp:9190-9195
Publication Date(Web):August 16, 2013
DOI:10.1021/jo401437w
A general atom-economical approach for the synthesis of α-halomethyl ketones is demonstrated through hydration of a wide range of haloalkynes. Other outstanding features include excellent yields from both alkyl- and aryl-substituted haloalkynes and wide functional group tolerance. This protocol is an alternative to conventional α-halogenation of ketones.


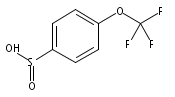
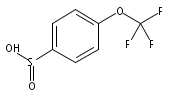

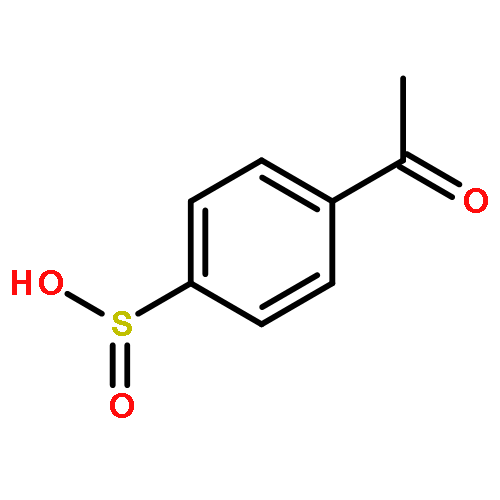
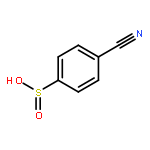





![Benzene,1,1'-[(1E)-1,2-ethenediylbis(sulfonyl)]bis[4-methyl-](http://img.cochemist.com/ccimg/15800/15717-50-7.png)
![Benzene,1,1'-[(1E)-1,2-ethenediylbis(sulfonyl)]bis[4-methyl-](http://img.cochemist.com/ccimg/15800/15717-50-7_b.png)
![Benzene,1,1'-[(1Z)-1,2-ethenediylbis(sulfonyl)]bis[4-methyl-](http://img.cochemist.com/ccimg/15700/15645-75-7.png)
![Benzene,1,1'-[(1Z)-1,2-ethenediylbis(sulfonyl)]bis[4-methyl-](http://img.cochemist.com/ccimg/15700/15645-75-7_b.png)



