Co-reporter:Shingo Atobe, Haruna Masuno, Motohiro Sonoda, Yuki Suzuki, Hiroyuki Shinohara, Satoshi Shibata, Akiya Ogawa
Tetrahedron Letters 2012 Volume 53(Issue 14) pp:1764-1767
Publication Date(Web):4 April 2012
DOI:10.1016/j.tetlet.2012.01.105
In the presence of 1-(2-pyridylethynyl)-2-(2-thienylethynyl)benzene as a ligand, the direct synthesis of alkynones has accomplished by a Pd-catalyzed coupling reaction of acid chlorides with terminal acetylenes under mild conditions.
Co-reporter:Hiroyuki Shinohara, Motohiro Sonoda, Shingo Atobe, Haruna Masuno, Akiya Ogawa
Tetrahedron Letters 2011 Volume 52(Issue 47) pp:6238-6241
Publication Date(Web):23 November 2011
DOI:10.1016/j.tetlet.2011.09.068
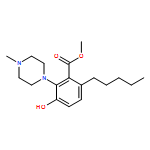
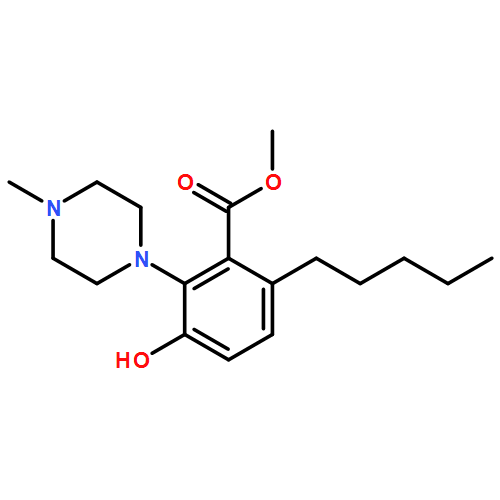
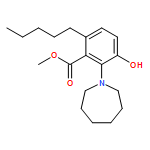
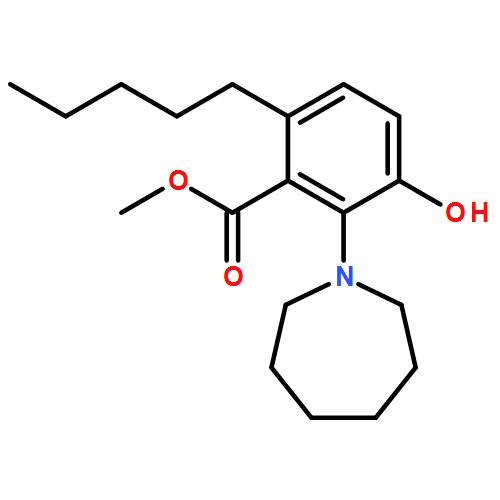
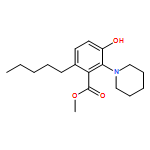
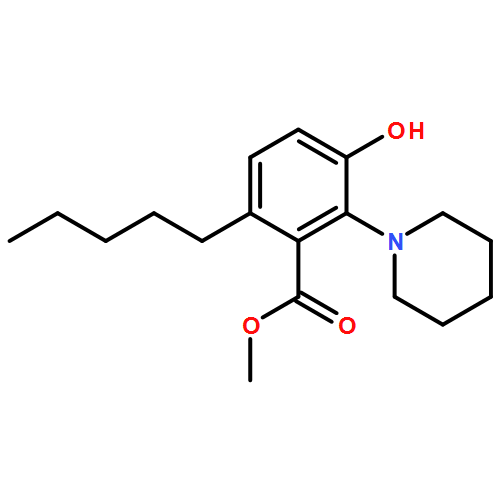
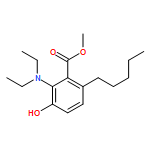
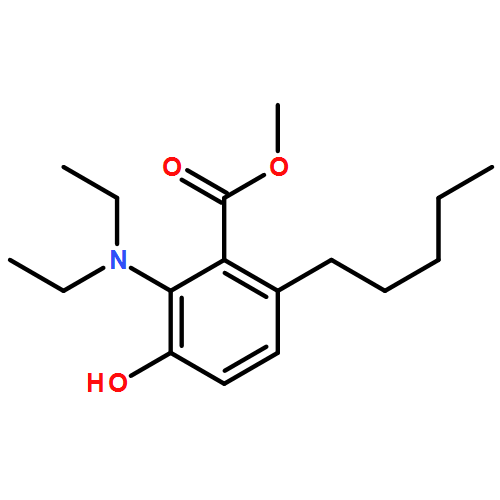
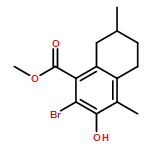
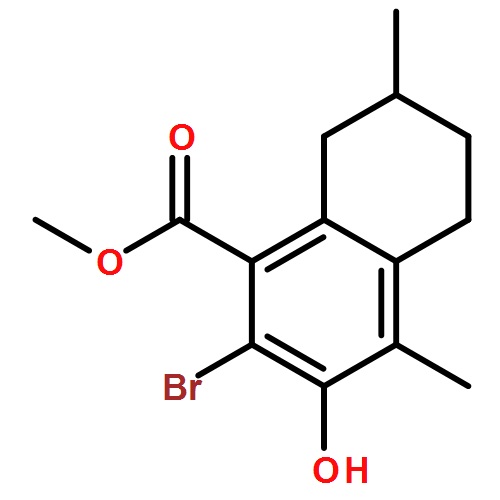
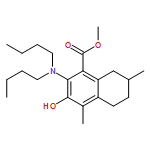
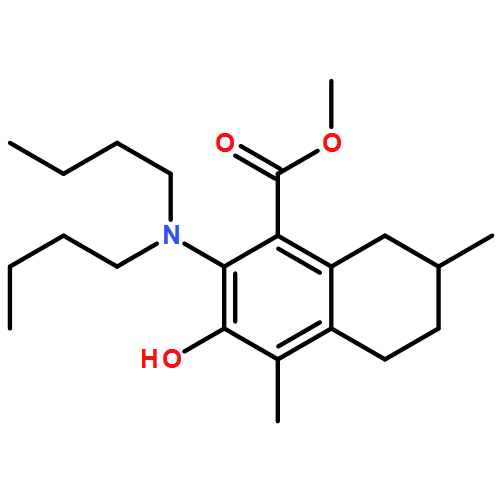
IrCl3·3H2O or FeCl3-catalyzed convenient synthesis of 3-hydroxyphthalates has been achieved by a Diels–Alder reaction of furans with dimethyl acetylenedicarboxylate, followed by ring-opening aromatization reaction of the Diels–Alder adducts, 7-oxabicyclo[2.2.1]hepta-2,5-diene derivatives. In addition, 7-azabicyclo[2.2.1]hepta-2,5-diene derivative, derived from N-Boc-pyrrole and dimethyl acetylenedicarboxylate, also converted into 3-aminophthalate derivative.


![7-Oxabicyclo[2.2.1]hepta-2,5-diene-2-carboxylic acid, 1-pentyl-3-(1-piperidinyl)-, methyl ester](/data/chemimg/3746700/1705593-84-5.png)
![7-Oxabicyclo[2.2.1]hepta-2,5-diene-2-carboxylic acid, 1-pentyl-3-(1-piperidinyl)-, methyl ester](/data/chemimg/3746700/1705593-84-5_b.png)
![7-Oxabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, 3-bromo-3-(dibutylamino)-1-pentyl-, methyl ester](/data/chemimg/3746700/1705593-83-4.png)
![7-Oxabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, 3-bromo-3-(dibutylamino)-1-pentyl-, methyl ester](/data/chemimg/3746700/1705593-83-4_b.png)
![Benzoic acid, 2-[bis(phenylmethyl)amino]-3-hydroxy-6-pentyl-, methyl ester](/data/chemimg/3746700/1705593-82-3.png)
![Benzoic acid, 2-[bis(phenylmethyl)amino]-3-hydroxy-6-pentyl-, methyl ester](/data/chemimg/3746700/1705593-82-3_b.png)
![Benzoic acid, 3-hydroxy-2-[methyl(phenylmethyl)amino]-6-pentyl-, methyl ester](/data/chemimg/3746700/1705593-81-2.png)
![Benzoic acid, 3-hydroxy-2-[methyl(phenylmethyl)amino]-6-pentyl-, methyl ester](/data/chemimg/3746700/1705593-81-2_b.png)