Co-reporter:Hongzhi Du, Mao Pang, Xiaoying Hou, Shengtao Yuan, Li Sun
Biomedicine & Pharmacotherapy 2017 Volume 90(Volume 90) pp:
Publication Date(Web):1 June 2017
DOI:10.1016/j.biopha.2017.04.023
Collagen is not only the most abundant protein providing the scaffold for assembly of the extracellular matrix (ECM), but also considered to be the “highway” for cancer cell migration and invasion depending on the different collagen organizations. The accumulation of stabilized collagen is enhanced by different covalent collagen cross-links, lysyl hydroxylases 2 (encoded by the PLOD2 gene) is the key enzyme mediating the formation of the stabilized collagen cross-link. Interestingly, PLOD2 is overexpressed in different cancers and closely related to a poor prognosis. To the best of our knowledge, only the mechanisms of PLOD2 regulated by HIF-1α, TGF-β and microRNA-26a/b have been elaborated. In addition, several pharmacologic inhibitors of PLOD2 have been confirmed to have an anti-metastasis effect. However, there have been no reviews about PLOD2 in cancer research published thus far. In brief, this review about PLOD2 will describe the function, regulatory mechanism, and the inhibitors of PLOD2 in cancer, suggesting the credible clinical evaluation of a prognostic signature in pathological examination and the possible development of therapeutic strategies targeting PLOD2 in the future.
Co-reporter:Debmalya Roy, Gao Ying Sheng, Semukunzi Herve, Evandro Carvalho, Arpan Mahanty, Shengtao Yuan, Li Sun
Biomedicine & Pharmacotherapy 2017 Volume 89(Volume 89) pp:
Publication Date(Web):1 May 2017
DOI:10.1016/j.biopha.2017.01.019
A growing interest has emerged in the field of studying the cross-talk between cancer cell cycle and metabolism. In this review, we aimed to present how metabolism and cell cycle are correlated and how cancer cells get energy to drive cell cycle. Cell proliferation and cell death largely depend on the metabolic activity of the cell. Cell cycle proteins, e.g. cyclin D, cyclin dependent kinase (CDK), some pro-apoptotic and anti-apoptotic proteins, and P53 have been shown to be regulated by metabolic crosstalk. Dysregulation of this cross-talk between metabolism and cell cycle leads to degenerative disorder(s) and cancer. It is not fully understood the actual reason of aberration between metabolism and cell cycle, but it is a hallmark of cancer research. Herein, we discussed the role of some regulatory molecules relative of cell cycle and metabolism and highlight how they control the function of each other. We also pointed out, current therapeutic opportunities and some additional crucial therapeutic targets on these fields that could be a breakthrough in cancer research.




![(3R,4aR,5S,6R,6aR,10R,10aS,10bR)-5-hydroxy-4a,6a,7,10b-tetramethyl-5'-oxo-1,2,4a,4',5,5',6,6a,9,10,10a,10b-dodecahydro-2'H-spiro[benzo[f]chromene-3,3'-furan]-6,10-diyl dinicotinate](http://img.cochemist.com/ccimg/1206900/1206805-30-2.png)
![(3R,4aR,5S,6R,6aR,10R,10aS,10bR)-5-hydroxy-4a,6a,7,10b-tetramethyl-5'-oxo-1,2,4a,4',5,5',6,6a,9,10,10a,10b-dodecahydro-2'H-spiro[benzo[f]chromene-3,3'-furan]-6,10-diyl dinicotinate](http://img.cochemist.com/ccimg/1206900/1206805-30-2_b.png)
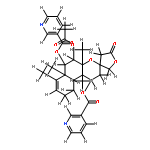

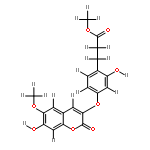

![6,8-diamino-7-chloro-1-methyl-2-oxo-1,2-dihydropyrrolo[4,3,2-de]quinoline-4-carboxamide](http://img.cochemist.com/ccimg/1096400/1096365-01-3.png)
![6,8-diamino-7-chloro-1-methyl-2-oxo-1,2-dihydropyrrolo[4,3,2-de]quinoline-4-carboxamide](http://img.cochemist.com/ccimg/1096400/1096365-01-3_b.png)
![1H-Pyrrolo[2,3-b]pyridine-2-butanenitrile](http://img.cochemist.com/ccimg/1006900/1006880-59-6.png)
![1H-Pyrrolo[2,3-b]pyridine-2-butanenitrile](http://img.cochemist.com/ccimg/1006900/1006880-59-6_b.png)


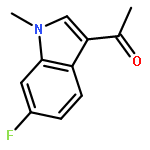



![1H-Pyrrolo[3,2-b]pyridine-2-butanenitrile](http://img.cochemist.com/ccimg/947400/947330-65-6.png)
![1H-Pyrrolo[3,2-b]pyridine-2-butanenitrile](http://img.cochemist.com/ccimg/947400/947330-65-6_b.png)