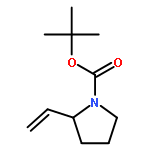
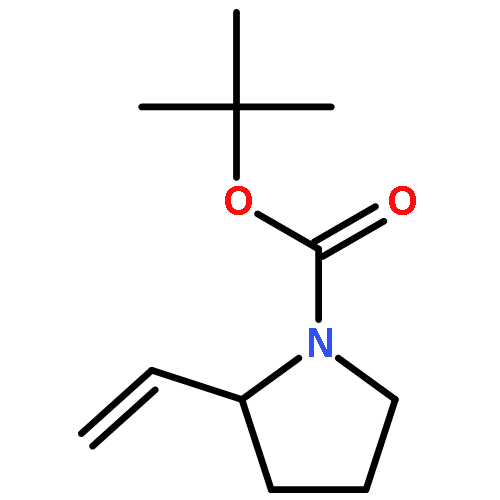
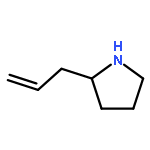
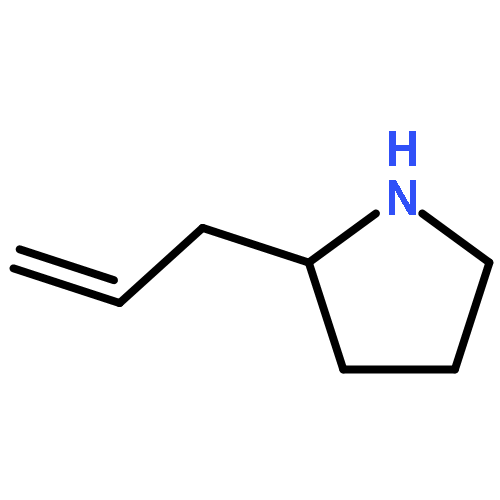
Co-reporter:Hao-Jie Rong, Jun-Jun Yao, Ji-Kun Li, and Jin Qu
The Journal of Organic Chemistry June 2, 2017 Volume 82(Issue 11) pp:5557-5557
Publication Date(Web):May 12, 2017
DOI:10.1021/acs.joc.7b00361
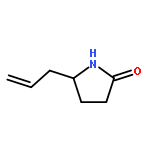
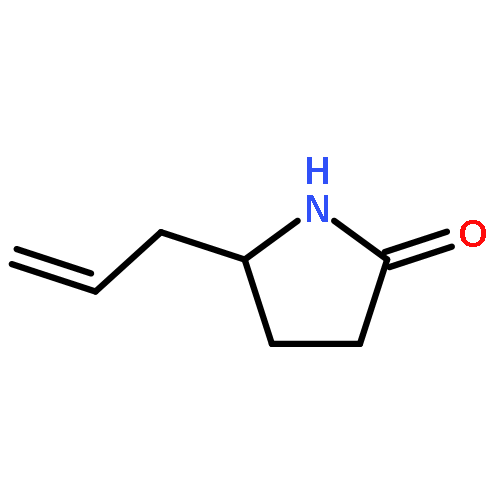
We previously reported an iterative synthesis of unsymmetrical 2,5-disubstituted pyrrolidines from pyrrolidine by two rounds of redox-triggered α-C–H functionalization. Although this approach can be used to introduce substituents at the 2- and 5-positions, it is lengthy because the redox auxiliary must be removed and then reinstalled. Therefore, we sought to develop a method to oxidize 2-functionalized pyrrolidine to cyclic N,O-acetal which could then react with a nucleophile for introduction of the 5-substituent. In this work, we found that molecular iodine can mediate the preferential oxidation of secondary over tertiary α-C–H bonds of α-substituted pyrrolidines to form cyclic N,O-acetals, improving the step economy of our previously reported method. With this strategy, (±)-preussin and its C(3) epimer were synthesized from (±)-pyrrolidin-3-ol.
Co-reporter:Hao-Jie Rong, Yong-Feng Cheng, Fan-Fan Liu, Shu-Jian Ren, and Jin Qu
The Journal of Organic Chemistry 2017 Volume 82(Issue 1) pp:532-540
Publication Date(Web):December 2, 2016
DOI:10.1021/acs.joc.6b02562
The late-stage oxidation of substituted pyrrolidines offers good flexibility for the construction of γ-lactam libraries, and especially in recent years the methods for functionalization of pyrrolidine have been available. We reported a new strategy for oxidation of pyrrolidines to γ-lactams: reaction of pyrrolidine with an o-benzoquinone gives an N,O-acetal by direct oxidation of the α-C–H bond of the pyrrolidine ring, and then the N,O-acetal is further oxidized by the o-benzoquinone to the γ-lactam. Because the first oxidation occurs selectively at the α-C–H of the pyrrolidine ring, oxidation-sensitive functional groups (allyl-, vinyl-, hydroxyl-, and amino groups) on pyrrolidine ring are unaffected. The synthetic utility of this novel method was demonstrated by the facile syntheses of (S)-vigabatrin and two analogues.
Co-reporter:Yan Tian; Xin Xu; Lin Zhang
Organic Letters 2016 Volume 18(Issue 2) pp:268-271
Publication Date(Web):January 7, 2016
DOI:10.1021/acs.orglett.5b03438
The use of an excess amount of tetraphenylphosphonium tetrafluoroborate in 1,1,1,3,3,3-hexafluoroisopropanol, which can stabilize the intermediate cation in the reaction, efficiently promoted epoxide-initiated cation–olefin polycyclization reactions with broad functional group tolerance and water and oxygen tolerance.
Co-reporter:Pei-Fang Li;Cheng-Bo Yi;Shu-Jian Ren
Advanced Synthesis & Catalysis 2016 Volume 358( Issue 13) pp:2088-2092
Publication Date(Web):
DOI:10.1002/adsc.201600246
Co-reporter:Yong-Feng Cheng, Hao-Jie Rong, Cheng-Bo Yi, Jun-Jun Yao, and Jin Qu
Organic Letters 2015 Volume 17(Issue 19) pp:4758-4761
Publication Date(Web):September 17, 2015
DOI:10.1021/acs.orglett.5b02298
By using o-benzoquinone as an internal oxidant, the regio- and diastereoselective functionalization of the secondary over the tertiary α-C–H bond of 2-substituted pyrrolidines is first realized. Subsequent intermolecular addition of a nucleophile to the generated N,O-acetal and cleavage of the aromatic substituent leads to 2,5-disubstituted pyrrolidines.
Co-reporter:Pei-Fang Li, Cheng-Bo Yi and Jin Qu
Organic & Biomolecular Chemistry 2015 vol. 13(Issue 17) pp:5012-5021
Publication Date(Web):18 Mar 2015
DOI:10.1039/C5OB00305A
In refluxing 9:1 (v/v) H2O–1,4-dioxane and without an additional catalyst, the rearrangements of various types of cyclopropyl carbinols were attempted. It was found that the reactions generally gave homoallylic alcohols in good to very high chemical yields. Rearrangements of bicyclic or tricyclic cyclopropyl carbinols readily gave the desired ring-expanded cyclic homoallylic alcohols which are difficult to synthesize by other means.
Co-reporter:Feng-Zhi Zhang, Yan Tian, Guo-Xing Li, and Jin Qu
The Journal of Organic Chemistry 2015 Volume 80(Issue 2) pp:1107-1115
Publication Date(Web):December 19, 2014
DOI:10.1021/jo502636d
Hot water, acting as a mildly acidic catalyst, efficiently promoted intramolecular direct nucleophilic substitution reactions of unsaturated alcohols with heteroatom or carbon nucleophiles. In a mixed solvent of water and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), polyene cyclizations using allylic alcohols as initiators gave the desired cyclized products, and in neat HFIP, a tricyclization reaction gave a tetracyclic product in 51% chemical yield.
Co-reporter:Li-Na Wang, Su-Li Shen and Jin Qu
RSC Advances 2014 vol. 4(Issue 58) pp:30733-30741
Publication Date(Web):17 Jul 2014
DOI:10.1039/C4RA03628J
1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) can act as both the solvent and the catalyst to promote the Pictet–Spengler reactions between tryptamine derivatives and aldehydes or activated ketones. For most substrates, removing the low boiling point HFIP by distillation directly afforded tetrahydro-β-carbolines in high yields.
Co-reporter:Yi-Jie Zuo and Jin Qu
The Journal of Organic Chemistry 2014 Volume 79(Issue 15) pp:6832-6839
Publication Date(Web):July 7, 2014
DOI:10.1021/jo500733v
It was widely reported that under the “on water” condition, various water-promoted organic reactions can proceed with very high speed. Thus, it is considered that the aqueous solubility of reactant is not an important issue in these reactions. Three types of water-promoted organic reactions were investigated in the current study to distinguish whether the reaction rate of an aqueous reaction was affected by the aqueous solubilities of the reactants. The results showed that, for a Diels–Alder reaction which was fast under the neat conditions, the aqueous solubilities of reactants had little influence on the reaction. However, for the reactions which proceeded slowly under the neat conditions, such as [2σ+2σ+2π] cycloaddition reactions and epoxide aminolysis reactions, the reactants with good aqueous solubilities proceeded fast in water. Poorly aqueous soluble reactants reacted slowly or did not react under the “on water” condition, and an appropriate amount of organic cosolvent was needed to make the reaction become efficient. This evidence suggested that for these two types of reactions, the dissolution of the reactants in water was required.
Co-reporter:Pei-Fang Li, Heng-Lu Wang, and Jin Qu
The Journal of Organic Chemistry 2014 Volume 79(Issue 9) pp:3955-3962
Publication Date(Web):April 9, 2014
DOI:10.1021/jo5004086
It was reported for the first time that hot water as a mildly acidic catalyst efficiently promoted 1,n-rearrangement (n = 3, 5, 7, 9) of allylic alcohols. In some cases, the rearrangement reactions joined isolated C–C double or triple bonds to generate conjugated polyene or enyne structure motifs. We used the 1,3-rearrangement reaction of an allylic alcohol in hot water as part of an attractive new strategy for construction of the polyene natural product navenone B by iterative use of a Grignard reaction, a 1,3-rearrangement of the resulting allylic alcohol, and subsequent oxidation of the rearranged product.
Co-reporter:Jin-Li Cao, Su-Li Shen, Peng Yang, and Jin Qu
Organic Letters 2013 Volume 15(Issue 15) pp:3856-3859
Publication Date(Web):July 19, 2013
DOI:10.1021/ol401581p
In refluxing water and without an additional catalyst, electron-rich phenols could react with alkynoic acids or alkynoates to provide coumarin structures. The skeletons of two natural pyranocoumarins, 5-methoxyseselin and alloxanthoxyletin, could be constructed (total yield up to 76%) in an aqueous multicomponent reaction in which isoprenyl acetate, propiolic acid, and phloroglucinol were simply mixed and refluxed in water.
Co-reporter:Dr. Zhao-Bing Xu ;Dr. Jin Qu
Chemistry - A European Journal 2013 Volume 19( Issue 1) pp:314-323
Publication Date(Web):
DOI:10.1002/chem.201202886
Abstract
During the studies of hydrolysis of epoxides in water, we found that the hydrolysis of (−)-α-pinene oxide at 20 °C gave enantiomerically pure trans-(−)-sobrerol, whereas the same reaction in water heated at reflux unexpectedly gave a racemic mixture of trans- and cis-sobrerol (trans/cis=6:4). We have examined this remarkable difference in detail and found that hot water, whose behavior is quite different compared with room- or high-temperature water, could promote SN1 solvolysis reactions of allylic alcohols and thus caused the racemization of trans-(−)-sobrerol. The effect of reaction temperature, the addition of organic co-solvent, and the concentration of the solute on the rate of the racemization of trans-(−)-sobrerol were further examined to understand the role that hot water played in the reaction. It was proposed that the catalytic effects of hot water are owing to its mild acidic characteristic, thermal activation, high ionizing power, and better solubility of organic reactant. Further investigation showed that the racemization of other chiral allylic/benzylic alcohols could efficiently proceed in hot water.
Co-reporter:Peng Yang, Pei-Fang Li, Jin Qu, and Liang-Fu Tang
Organic Letters 2012 Volume 14(Issue 15) pp:3932-3935
Publication Date(Web):July 20, 2012
DOI:10.1021/ol3016836
The C2-symmetric (−)-glabrescol was synthesized in two steps from (10S,11R)-dihydroxy-10,11-dihydrosqualene or squalene with 50% or 10% overall yields, respectively. These highly efficient and biomimetic syntheses employed a base-promoted middle-to-terminal double epoxide-opening cascade, which constructs the five tetrahydrofuran rings in glabrescol in one operation.
Co-reporter:Guo-Xing Li and Jin Qu
Chemical Communications 2012 vol. 48(Issue 44) pp:5518-5520
Publication Date(Web):17 Apr 2012
DOI:10.1039/C2CC31735D
Enantioselective Friedel–Crafts reactions between phenols and N-tosylaldimines were developed using a bifunctional catalyst readily prepared from L-leucine. The chiral benzylic amine products were obtained in high yields (up to 96% yield) and good to high enantiomeric excesses (up to 95% ee).
Co-reporter:Zhao-Bing Xu
Chinese Journal of Chemistry 2012 Volume 30( Issue 5) pp:1133-1136
Publication Date(Web):
DOI:10.1002/cjoc.201100455
Abstract
The efficient hydrolytic kinetic separation of trans/cis-(R)-(+)-limonene oxides was realized in a 1:1 mixed solvent of water and 1,4-dioxane without additional catalyst. Optically pure trans-(R)-(+)-limonene oxide was recovered in high yield (77%).
Co-reporter:Peng Chen and Jin Qu
The Journal of Organic Chemistry 2011 Volume 76(Issue 9) pp:2994-3004
Publication Date(Web):March 15, 2011
DOI:10.1021/jo200403g
Synthetic oligopeptides as mimics of enzymes have been increasingly exploited as catalysts for asymmetric reactions, but highly effective oligopeptide catalysts with relatively low molecular weight are still in great demand. In this paper, we showed the conformational engineering of the β-hairpin-forming tetrapeptide 4 which was first reported by Miller’s group as the catalyst for the asymmetric acyl transfer reaction of trans-2-(N-acetylamino)cyclohexan-1-ol (krel = 28). Through our backbone modification strategy, thioamide and sulfonamide as the isosteres of amide were introduced in the β-hairpin secondary structure. The thioxo peptides also adopt β-hairpin conformations as the oxopeptide supported by the combined use of NMR, IR, and X-ray techniques. Thioxo tetrapeptide 14 formed a more constrained β-hairpin conformation and therefore delivered much higher enantioselectivity (krel = 109) in the same reaction. Moreover, the examination of the conformational changes of tetrapeptide 8 upon the protonation of the Nπ-methylhistidine moiety provided evidence to explain the variation of its catalytic efficiency in the asymmetric acyl-transfer reaction.
Co-reporter:ZhaoBing Xu
Science China Chemistry 2011 Volume 54( Issue 11) pp:1718-1725
Publication Date(Web):2011 November
DOI:10.1007/s11426-011-4323-x
Ring opening of extremely hydrophobic epoxides with water, amines, sodium azide and thiophenol was realized in the mixture solvent of water and 1, 4-dioxane under reflux condition. Hot water was believed to act as a mild Brønsted acid catalyst in the epoxide-opening reactions.
Co-reporter:Guo-Xing Li and Jin Qu
Chemical Communications 2010 vol. 46(Issue 15) pp:2653-2655
Publication Date(Web):10 Mar 2010
DOI:10.1039/B926684D
The stereoselective intra- and intermolecular Friedel–Crafts alkylation of electron-rich arenes with epoxides can take place in refluxing 1,1,1,3,3,3-hexafluoroisopropanol owing to its weak acidity and high ionizing power.
Co-reporter:Jia Wang, Yan-Liang Liang and Jin Qu
Chemical Communications 2009 (Issue 34) pp:5144-5146
Publication Date(Web):31 Jul 2009
DOI:10.1039/B910239F
A general protocol for removing Boc groups from various types of nitrogen is reported and a preliminary investigation of the reaction mechanism indicates that water acts as a dual acid/base catalyst at elevated temperature.
Co-reporter:Guo-Xing Li and Jin Qu
Chemical Communications 2012 - vol. 48(Issue 44) pp:NaN5520-5520
Publication Date(Web):2012/04/17
DOI:10.1039/C2CC31735D
Enantioselective Friedel–Crafts reactions between phenols and N-tosylaldimines were developed using a bifunctional catalyst readily prepared from L-leucine. The chiral benzylic amine products were obtained in high yields (up to 96% yield) and good to high enantiomeric excesses (up to 95% ee).
Co-reporter:Pei-Fang Li, Cheng-Bo Yi and Jin Qu
Organic & Biomolecular Chemistry 2015 - vol. 13(Issue 17) pp:NaN5021-5021
Publication Date(Web):2015/03/18
DOI:10.1039/C5OB00305A
In refluxing 9:1 (v/v) H2O–1,4-dioxane and without an additional catalyst, the rearrangements of various types of cyclopropyl carbinols were attempted. It was found that the reactions generally gave homoallylic alcohols in good to very high chemical yields. Rearrangements of bicyclic or tricyclic cyclopropyl carbinols readily gave the desired ring-expanded cyclic homoallylic alcohols which are difficult to synthesize by other means.
Co-reporter:Jia Wang, Yan-Liang Liang and Jin Qu
Chemical Communications 2009(Issue 34) pp:NaN5146-5146
Publication Date(Web):2009/07/31
DOI:10.1039/B910239F
A general protocol for removing Boc groups from various types of nitrogen is reported and a preliminary investigation of the reaction mechanism indicates that water acts as a dual acid/base catalyst at elevated temperature.
Co-reporter:Guo-Xing Li and Jin Qu
Chemical Communications 2010 - vol. 46(Issue 15) pp:NaN2655-2655
Publication Date(Web):2010/03/10
DOI:10.1039/B926684D
The stereoselective intra- and intermolecular Friedel–Crafts alkylation of electron-rich arenes with epoxides can take place in refluxing 1,1,1,3,3,3-hexafluoroisopropanol owing to its weak acidity and high ionizing power.


![OXIRANE, 2,2-DIMETHYL-3-[(3E)-3-METHYL-6-(3-METHYLPHENYL)-3-HEXENYL]-](http://img.cochemist.com/ccimg/866500/866474-71-7.png)
![OXIRANE, 2,2-DIMETHYL-3-[(3E)-3-METHYL-6-(3-METHYLPHENYL)-3-HEXENYL]-](http://img.cochemist.com/ccimg/866500/866474-71-7_b.png)