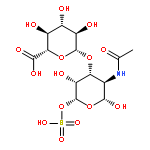
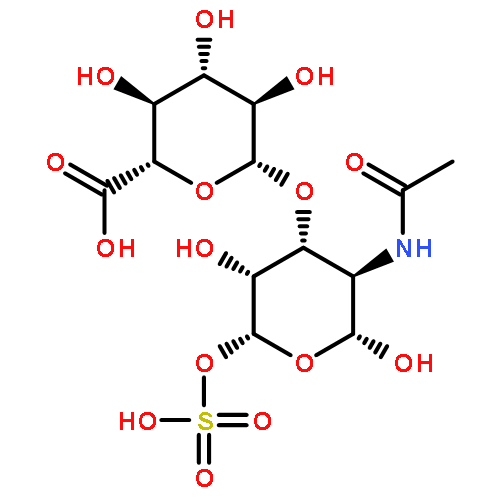
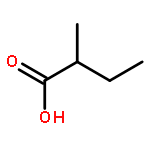
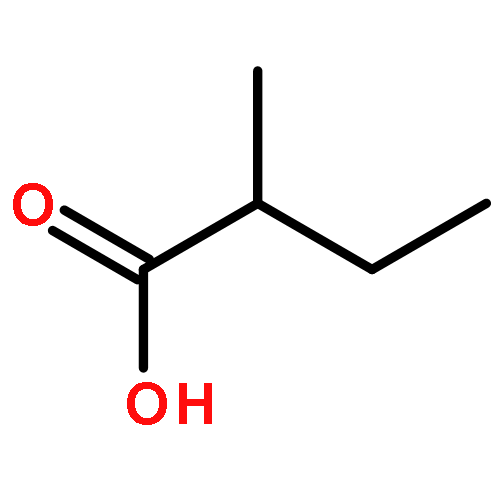
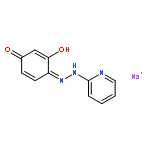
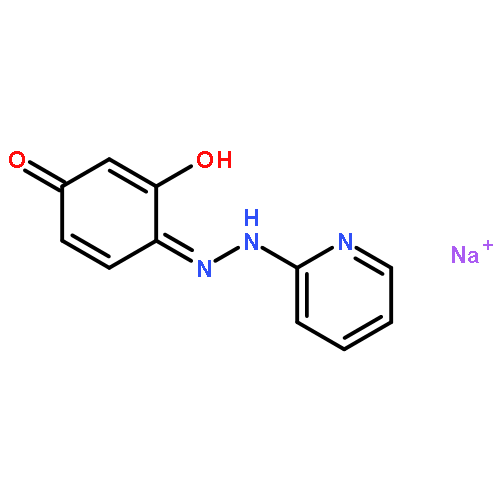
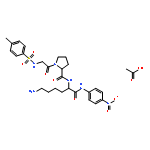
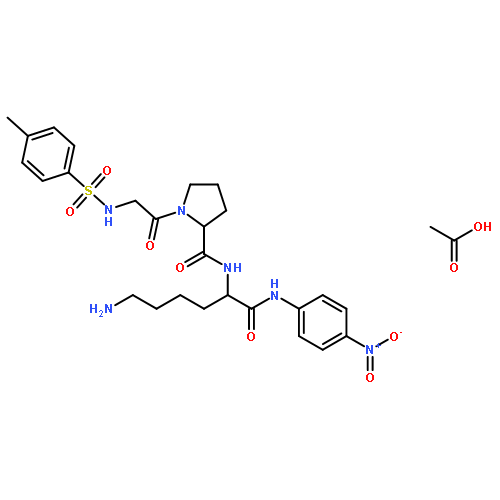
Co-reporter:Jin-Jin LU, Xue-Wen HU, Ping LI, Jun CHEN
Chinese Journal of Natural Medicines 2017 Volume 15, Issue 7(Volume 15, Issue 7) pp:
Publication Date(Web):1 July 2017
DOI:10.1016/S1875-5364(17)30082-1
Si-Miao-Wan (SMW), a tradiational Chinese medicinal formula consisting of Atractylodis Rhizoma, Phellodendri Chinensis Cortex, Coicis Semen, and Achyranthis Bidentatae Radix, has been used for the treatment of gout and gouty arthritis for many years. In the present study, a liquid chromatography quadrupole-time-of-flight mass spectrometry (LC-Q-TOF/MS) method was established to identify the multiple constituents of SMW and its metabolites in rat biological samples after oral administration. A total of 48 compounds in SMW, including 21 alkaloids, 12 organic acids, 2 terpenes, 3 lactones, 2 phytosterols, and 8 other compounds, were tentatively characterized with the diagnostic-ion filtering strategy. Based on the diagnostic ions applied to identify compounds in SMW, 28 prototype compounds and 10 metabolic compounds were detected in the biological samples. This was the first comprehensive drug metabolism investigation of SMW in rats. The developed method could be a useful means for identifying the multi-components in SMW and the metabolic components. The results may help explore the possible metabolic processes and mechanism of action for SMW in vivo.
Co-reporter:Xue-Yan Li, Hong-Jin Tang, Liu Zhang, Lin Yang, Ping Li, Jun Chen
Journal of Chromatography B 2017 Volumes 1068–1069(Volumes 1068–1069) pp:
Publication Date(Web):15 November 2017
DOI:10.1016/j.jchromb.2017.10.011
•A method for screening minor active components from complex plants was developed.•The selective knockout method is more efficient and greener.•From S. miltiorrhiza, 21 minor components were first discovered as Nrf2 activators.Natural products have been recognized to play an invaluable role in drug discovery. However, efficient discovery of minor active constituents from natural sources is challenging due to the low abundance and complex matrices. In this study, we developed a selective knockout method to discover minor bioactive components from complex phytochemical mixtures, using a Chinese medicine as an example. Based on the chromatographic fingerprint, six major components in the ethyl acetate extract of the root of Salvia miltiorrhiza (EASM) were selectively knocked out via high-resolution peak fraction (HRPF) approach. The remaining extract was automatically enriched and fractionated to generate a chemical library consisting of 62 minor components with contents less than 3‰. Simultaneously, a parallel mass-spectrometry (MS) analysis was performed to ensure purity and to characterize the structure of the compound in each fraction. Via an antioxidant response element (ARE)-driven luciferase reporter system, 33 minor components were screened out as nuclear factor erythroid 2-related factor 2 (Nrf2) activators and 30 components were identified. Here, the Nrf2 activation activities of 21 components have been reported for the first time. Different from the existing methods for discovery of active compounds from natural products, in the developed method of this manuscript, the major components are selectively removed, and the fractions of the minor components are prepared after several times of preparative HPLC enrichment by high-resolution peak fraction approach. It improves the prospective discovery of minor active components from complex medicinal herbs.
Co-reporter:Weiwei Tang;Bixia Huang;Jiancheng Wang;Lin An;Huailing Zhong;Hua Yang;Ping Li
RSC Advances (2011-Present) 2017 vol. 7(Issue 68) pp:43005-43013
Publication Date(Web):2017/09/04
DOI:10.1039/C7RA07927C
Protease-activated receptor 1 (PAR-1) antagonists strongly inhibit thrombin-induced platelet aggregation and are proved to be effective as anti-thrombotic drugs. Traditional screening assays for PAR-1 antagonists require molecular labeling or cell engineering technique. Here, a label-free approach was developed for the screening of active compounds targeting PAR-1 through monitoring integrated live-cell responses in whole cells. To characterize the cellular response, the cellular dynamic mass redistribution (DMR) was detected with a resonant waveguide grating (RWG) biosensor using PAR-1 known agonists and antagonists. The human epidermoid carcinoma A431 cell line was selected to establish a cell phenotypic profiling model for screening the PAR-1 antagonists from 80 natural products. Results showed that five compounds were screened out as candidate bioactive compounds. Two compounds, parthenolide and sanguinarine, were identified to possess anti-platelet aggregation activities in vitro. These results indicate that the label-free DMR screening approach is effective and useful for screening bioactive compounds targeting PAR-1.
Co-reporter:Hong-Jin Tang, Xiao-Wei Zhang, Lin Yang, Wei Li, Jia-Huang Li, Jin-Xin Wang, Jun Chen
European Journal of Medicinal Chemistry 2016 Volume 124() pp:637-648
Publication Date(Web):29 November 2016
DOI:10.1016/j.ejmech.2016.08.019
•2-arylbenzo[b]furan derivatives were synthesized as non-purine XO inhibitors based on salvianolic acid C.•The XO inhibitory potency and antioxidant activity of these derivatives were evaluated.•The structure-activity relationships were preliminary investigated.•Molecular modeling studies were performed to explore the potential binding mechanisms.Xanthine oxidase (XO) is the key enzyme in humans which is related to a variety of diseases such as gout, hyperuricemia and cardiovascular diseases. In this work, a series of 2-arylbenzo[b]furan derivatives were synthesized based on salvianolic acid C, and they were evaluated for xanthine oxidase inhibitory and antioxidant activities. Compounds 5b, 6a, 6e and 6f showed potent xanthine oxidase inhibitory activities with IC50 values ranging from 3.99 to 6.36 μM, which were comparable with that of allopurinol. Lineweaver-Burk plots analysis revealed that the representative derivative 6e could bind to either xanthine oxidase or the xanthine oxidase-xanthine complex, which exhibited a mixed-type competitive mechanism. A DPPH radical scavenging assay showed most of the hydroxyl-functionalized 2-arylbenzo[b]furan derivatives possessed the potent antioxidant activity, which was further validated on LPS-stimulated RAW 264.7 macrophages model. The structure-activity relationships were preliminary analyzed and indicated that the structural skeleton of 2-arylbenzo[b]furan and phenolic hydroxyl groups played an important role in maintaining xanthine oxidase inhibitory effect and antioxidant property for the series of derivatives. Meanwhile, molecular docking studies were performed to further confirm the structure-activity relationships and investigate the proposed binding mechanisms of compounds 5d, 6d and 10d binding to the protein.