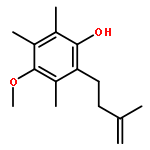
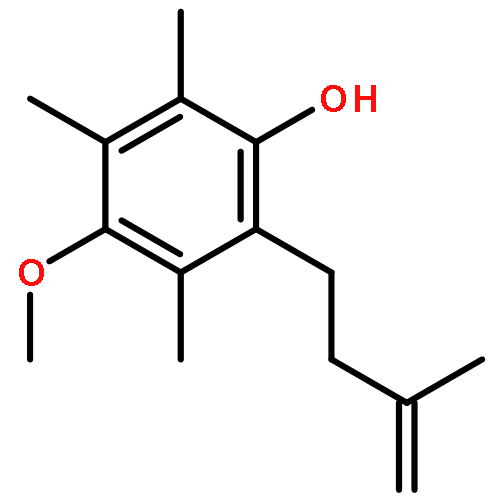
Co-reporter:Jiawang Liu; Zhaobin Han; Xiaoming Wang; Zheng Wang;Kuiling Ding
Journal of the American Chemical Society 2015 Volume 137(Issue 49) pp:15346-15349
Publication Date(Web):September 17, 2015
DOI:10.1021/jacs.5b07764
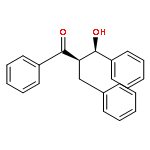
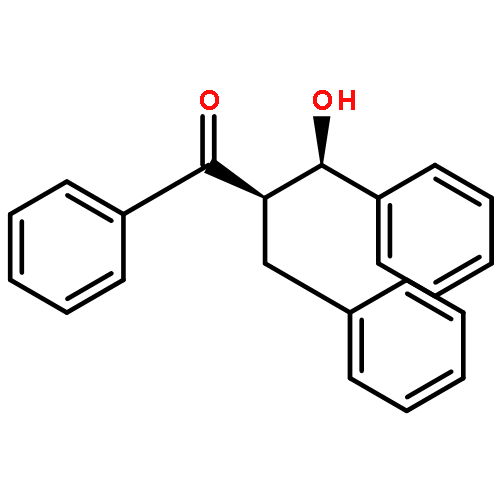
An enantioselective alkoxycarbonylation–amination cascade process of terminal allenes with CO, methanol, and arylamines has been developed. It proceeds under mild conditions (room temperature, ambient pressure CO) via oxidative Pd(II) catalysis using an aromatic spiroketal-based diphosphine (SKP) as a chiral ligand and a Cu(II) salt as an oxidant and affords a wide range of α-methylene-β-arylamino acid esters (36 examples) in good yields with excellent enantioselectivity (up to 96% ee) and high regioselectivity (branched/linear > 92:8). Preliminary mechanistic studies suggested that the reaction is likely to proceed through alkoxycarbonylpalladation of the allene followed by an amination process. The synthetic utility of the protocol is showcased in the asymmetric construction of a cycloheptene-fused chiral β-lactam.

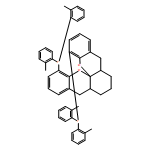
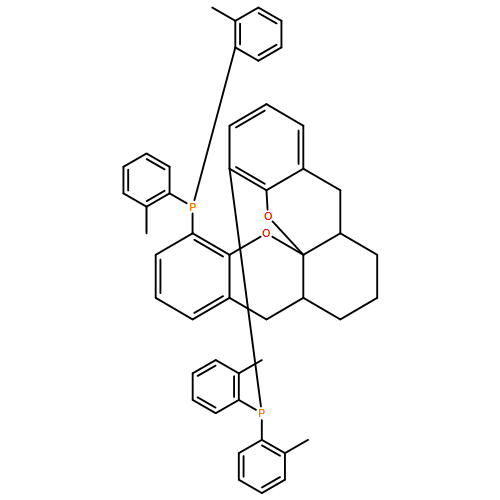
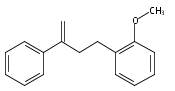
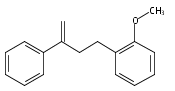
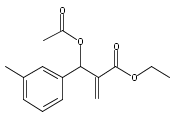
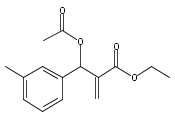
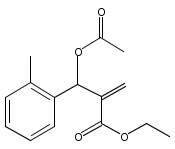

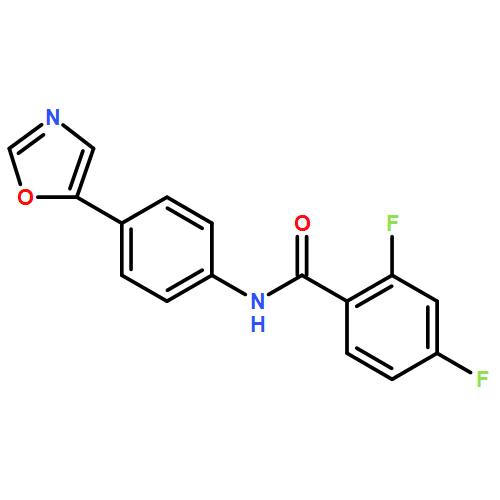
![Phosphine,1,1'-[(1R)-2,2',3,3'-tetrahydro-1,1'-spirobi[1H-indene]-7,7'-diyl]bis[1,1-diphenyl-(9CI)](/data/chemimg/1006900/917377-74-3.png)
![Phosphine,1,1'-[(1R)-2,2',3,3'-tetrahydro-1,1'-spirobi[1H-indene]-7,7'-diyl]bis[1,1-diphenyl-(9CI)](/data/chemimg/1006900/917377-74-3_b.png)