Co-reporter: James B. Gerken and Shannon S. Stahl
pp: 234
Publication Date(Web):July 15, 2015
DOI: 10.1021/acscentsci.5b00163
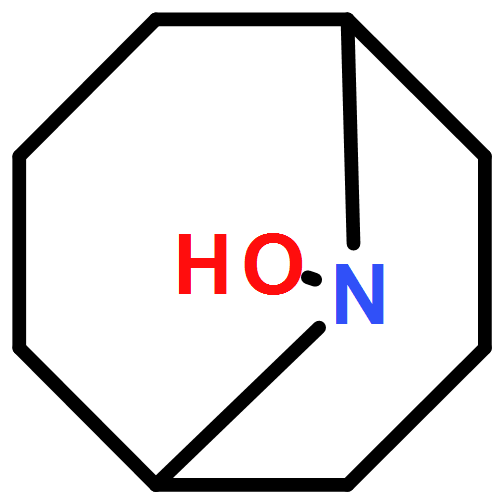
Efficient reduction of O2 to water is a central challenge in energy conversion and many aerobic oxidation reactions. Here, we show that the electrochemical oxygen reduction reaction (ORR) can be achieved at high potentials by using soluble organic nitroxyl and nitrogen oxide (NOx) mediators. When used alone, neither organic nitroxyls, such as 2,2,6,6-tetramethyl-1-piperidinyl-N-oxyl (TEMPO), nor NOx species, such as sodium nitrite, are effective ORR mediators. The combination of nitroxyl/NOx species, however, mediates sustained O2 reduction with overpotentials as low as 300 mV in acetonitrile containing trifluoroacetic acid. Mechanistic analysis of the coupled redox reactions supports a process in which the nitrogen oxide catalyst drives aerobic oxidation of a nitroxyl mediator to an oxoammonium species, which then is reduced back to the nitroxyl at the cathode. The electrolysis potential is dictated by the oxoammonium/nitroxyl reduction potential. The overpotentials accessible with this ORR system are significantly lower than widely studied molecular metal-macrocycle ORR catalysts and benefit from the mechanism-based specificity for four-electron reduction of oxygen to water mediated by NOx species, together with kinetically efficient reduction of oxidized NOx species by TEMPO and other organic nitroxyls.