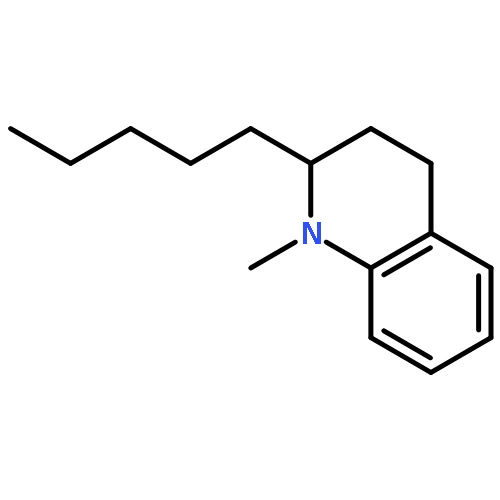
A new synthesis of (−)-angustureine is described, featuring an annulation and fragmentation sequence that formally results in alkynylation of a β-amino ester. The six-step sequence from a readily available chiral β-amino ester to the natural product was achieved in 46% yield, with no protecting groups and three purifications.