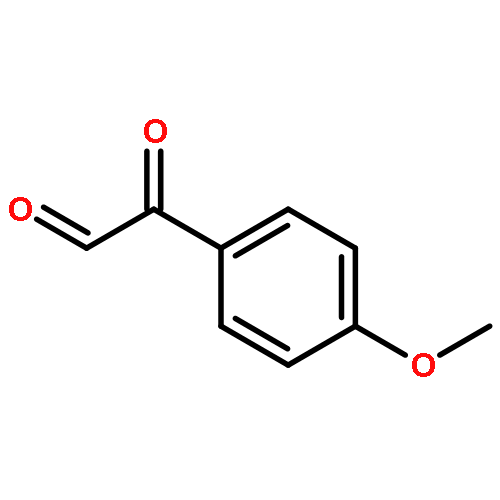
The following article describes a concise synthesis of a collection of 4,5-dihydro-1H-benzo[e][1,4]diazepines fused to a hydantoin ring. Molecular complexity and biological relevance are high and structures are generated in a mere three steps, employing the Ugi reaction to assemble diversity reagents. The protocol represents a novel UDC (Ugi-deprotect-cyclize) strategy employed in the Ugi-5-component CO2-mediated condensation, followed by further cyclization under basic conditions, to afford the fused hydantoin. Mechanistic caveats, dependent on the aldehydes of choice will be revealed and a facile oxidation of the final products to imidazolidenetriones is briefly discussed.The article describes a concise synthesis of a collection of 4,5-dihydro-1H-benzo[e][1,4]diazepines fused to a hydantoin ring, generated in a mere three steps. The protocol employs the Ugi-5-component CO2-mediated condensation, benzodiazepine formation promoted by acidic conditions and basic treatment to afford the fused hydantoin. Mechanistic caveats, dependent on the aldehydes of choice will be revealed and a facile oxidation of the final products to imidazolidenetriones is briefly discussed.