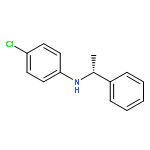
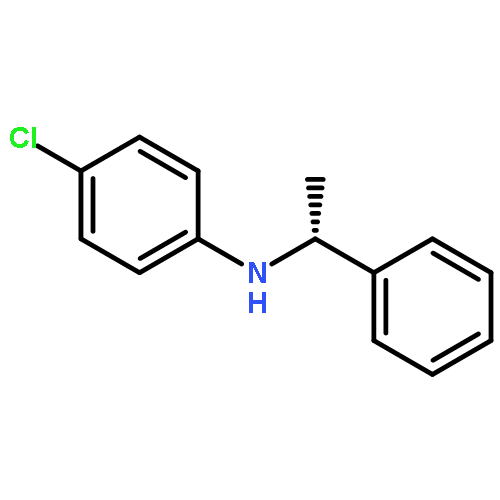
Co-reporter:Yongbing Liu and Haifeng Du
Journal of the American Chemical Society May 8, 2013 Volume 135(Issue 18) pp:6810-6813
Publication Date(Web):April 22, 2013
DOI:10.1021/ja4025808
This paper describes a highly enantioselective metal-free hydrogenation of imines using chiral dienes as “ligands” for the generation of catalysts with HB(C6F5)2 by hydroboration in situ to furnish a variety of chiral amines with up to 89% ee, which provides a practical strategy for the development of novel chiral frustrated Lewis pairs for asymmetric hydrogenation.
Co-reporter:Songlei Li, Wei Meng, and Haifeng Du
Organic Letters May 19, 2017 Volume 19(Issue 10) pp:
Publication Date(Web):May 1, 2017
DOI:10.1021/acs.orglett.7b00935
An asymmetric transfer hydrogenation of 2,3-disubstituted quinoxalines using a chiral frustrated Lewis pair of Piers’ borane and (R)-tert-butylsulfinamide as the catalyst with ammonia borane as the hydrogen source has been successfully realized. For 2-alkyl-3-arylquinoxaline substrates, cis-tetrahydroquinoxalines were obtained as the predominant products in high yields with 77–86% ee. In contrast, trans isomers were often furnished as major products for the reactions of 2,3-dialkylquinoxalines with up to >99% ee.
Co-reporter:Zhaoqun Liu, Xiangqing Feng, and Haifeng Du
Organic Letters June 15, 2012 Volume 14(Issue 12) pp:3154-3157
Publication Date(Web):June 6, 2012
DOI:10.1021/ol301248d
A simple chiral sulfur−alkene hybrid ligand has proven to be highly effective for rhodium-catalyzed formal cycloaddition reactions of racemic butadiene monoxide and imines to furnish spirooxindole oxazolidines or 1,3-oxazolidines with high yields and stereoselectivities. A possible dynamic kinetic resolution as well as a kinetic resolution is considered to be involved in this catalytic process.
Co-reporter:Xiaoyu Ren
Journal of the American Chemical Society 2016 Volume 138(Issue 3) pp:810-813
Publication Date(Web):January 11, 2016
DOI:10.1021/jacs.5b13104
A highly enantioselective hydrosilylation of 1,2-dicarbonyl compounds was successfully realized for the first time utilizing the combination of tricyclohexylphosphine and chiral alkenylborane derived in situ from diyne as a frustrated Lewis pair catalyst. A variety of optically active α-hydroxy ketones and esters were obtained in 52–98% yields with 86–99% ee’s.
Co-reporter:Songlei Li, Gen Li, Wei Meng, and Haifeng Du
Journal of the American Chemical Society 2016 Volume 138(Issue 39) pp:12956-12962
Publication Date(Web):September 8, 2016
DOI:10.1021/jacs.6b07245
Inspired by the zwitterion species generated from the splitting of H2 by frustrated Lewis pairs, we put forward a novel frustrated Lewis pair by the combination of Hδ- and Hδ+ incorporated Lewis acid and base together. Piers’ borane and chiral tert-butylsulfinamide were chosen as the FLP, and a metal-free asymmetric transfer hydrogenation of imines was realized with high enantioselectivities. Significantly, with ammonia borane as hydrogen source, a catalytic asymmetric reaction using 10 mol % of Piers’ borane, chiral tert-butylsulfinamide, and pyridine additive, has been successfully achieved to furnish optically active amines in 78–99% yields with 84–95% ee’s. Experimental and theoretical mechanistic studies reveal an interesting 8-membered ring hydrogen transfer transition state and an expected regeneration of reactive species with ammonia borane. Accordingly, a plausible catalytic pathway for this reaction is depicted.
Co-reporter:Qiwen Zhou, Lanqiong Zhang, Wei Meng, Xiangqing Feng, Jing Yang, and Haifeng Du
Organic Letters 2016 Volume 18(Issue 20) pp:5189-5191
Publication Date(Web):September 28, 2016
DOI:10.1021/acs.orglett.6b02610
With the use of ammonia borane as a hydrogen source, a borane catalyzed metal-free transfer hydrogenation of pyridines was successfully realized for the first time to furnish a variety of piperidines in 44–88% yields with moderate to excellent cis-selectivities. The ease in handling without requiring high pressure H2 makes this transfer hydrogenation practical and useful.
Co-reporter:Wei Wang, Wei Meng and Haifeng Du
Dalton Transactions 2016 vol. 45(Issue 14) pp:5945-5948
Publication Date(Web):03 Dec 2015
DOI:10.1039/C5DT03872C
This paper describes the first metal-free hydrogenation of 3,6-diarylpyridazines, which was successfully realized using B(C6F5)3 as a catalyst. A variety of 1,4,5,6-tetrahydropyridazine derivatives were furnished in 85–95% yields.
Co-reporter:Wei Wang, Xiangqing Feng and Haifeng Du
Organic & Biomolecular Chemistry 2016 vol. 14(Issue 28) pp:6683-6686
Publication Date(Web):16 Jun 2016
DOI:10.1039/C6OB01172A
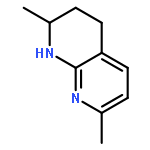
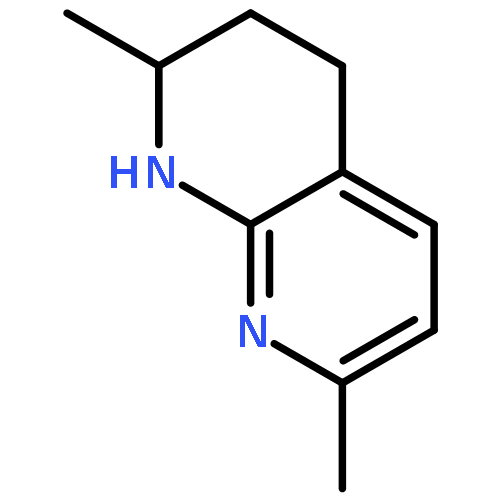
Metal-free hydrogenation of 2,7-disubstituted 1,8-naphthyridines was successfully realized for the first time using in situ generated borane catalysts under mild conditions to furnish 1,2,3,4-tetrahydro-1,8-naphthyridine derivatives in 83–98% yields. Significantly, up to 74% ee was achieved for the corresponding asymmetric hydrogenation reactions.
Co-reporter:Xiaoyu Ren, Gen Li, Simin Wei, and Haifeng Du
Organic Letters 2015 Volume 17(Issue 4) pp:990-993
Publication Date(Web):February 3, 2015
DOI:10.1021/acs.orglett.5b00085
A facile development of chiral alkenylboranes by the hydroboration of chiral diynes with Piers’ borane was successfully achieved for the first time. With the combination of the in situ generated chiral alkenylboranes and tri-tert-butylphosphine as frustrated Lewis pair catalysts, the metal-free asymmetric hydrogenation of silyl enol ethers was realized to furnish a wide range of optically active secondary alcohols in high yields and up to 99% ee.
Co-reporter:Zhenhua Zhang and Haifeng Du
Organic Letters 2015 Volume 17(Issue 11) pp:2816-2819
Publication Date(Web):May 11, 2015
DOI:10.1021/acs.orglett.5b01240
A highly enantioselective cis-hydrogenation of 2,3,4-trisubstituted quinolines has been realized for the first time using chiral borane catalysts generated in situ from chiral dienes. A variety of tetrahydroquinoline derivatives containing three contiguous stereogenic centers were obtained in 76–99% yields with 82–99% ee’s.
Co-reporter:Xiaxia Zhu and Haifeng Du
Organic Letters 2015 Volume 17(Issue 12) pp:3106-3109
Publication Date(Web):May 28, 2015
DOI:10.1021/acs.orglett.5b01380
A highly stereoselective metal-free hydrogenation of vicinal diimines has been successfully realized for the first time using 5–10 mol % of Piers’ borane as a catalyst under mild conditions, and a variety of cis-1,2-diamines were obtained in 92–99% yields. The current work provides a novel and efficient approach for the synthesis of vicinal diamines.
Co-reporter:Zhenhua Zhang and Haifeng Du
Organic Letters 2015 Volume 17(Issue 24) pp:6266-6269
Publication Date(Web):December 10, 2015
DOI:10.1021/acs.orglett.5b03307
A metal-free hydrogenation of 2,4-disubstituted quinolines was realized for the first time using chiral diene derived borane catalysts to furnish the corresponding tetrahydroquinolines in 75–98% yields with 95/5−99/1 dr’s and 86–98% ee’s. This catalytic system was also effective for 2,3-disubstituted quinolines to give moderate to good ee’s.
Co-reporter:Gen Li, Xiangqing Feng and Haifeng Du
Organic & Biomolecular Chemistry 2015 vol. 13(Issue 20) pp:5826-5830
Publication Date(Web):16 Apr 2015
DOI:10.1039/C5OB00663E
Isatins and their derivatives are important functional moities and building blocks in pharmaceutical and synthetic chemistry. Numerous enantioselective transformations at the C-3 carbonyl group have been well developed. However, the asymmetric substitution reaction with isatins and their derivatives as nucleophiles based on the free N–H groups has been less studied due to the relatively weaker nucleophilicity resulting from the two electron-withdrawing carbonyl groups. In this paper, a palladium-catalyzed asymmetric allylic amination of racemic butadiene monoxide with isatin derivatives using a chiral phosphoramidite olefin hybrid ligand has been successfully developed under mild conditions. A variety of chiral amino alcohols were afforded in 55–87% yields with 10/1->20/1 regioselectivity ratios and 80–97% ees.
Co-reporter:Gen Li, Yongbing Liu and Haifeng Du
Organic & Biomolecular Chemistry 2015 vol. 13(Issue 10) pp:2875-2878
Publication Date(Web):19 Jan 2015
DOI:10.1039/C5OB00009B
A catalytic metal-free hydrogenation of naphthylamines using B(C6F5)3 as a catalyst was successfully achieved under mild conditions for the first time to furnish a variety of tetrahydronaphthylamines in 88–99% yields.
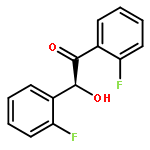
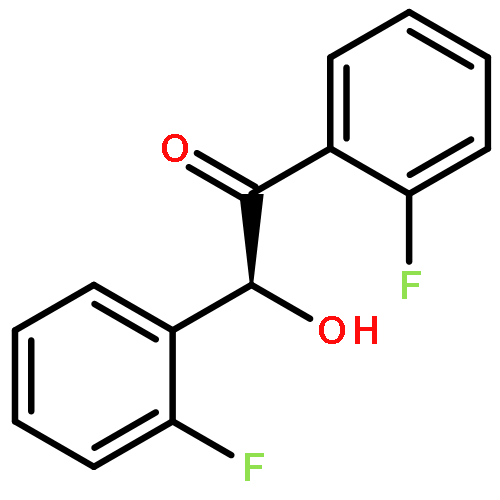
Co-reporter:Xiaxia Zhu and Haifeng Du
Organic & Biomolecular Chemistry 2015 vol. 13(Issue 4) pp:1013-1016
Publication Date(Web):28 Nov 2014
DOI:10.1039/C4OB02419B
An enantioselective hydrosilylation of imines was successfully achieved using a chiral borane catalyst generated by the in situ hydroboration of a binaphthyl-based chiral diene with Piers’ borane HB(C6F5)2 to furnish a variety of optically active amines in 70% to >99% yields and 44–82% ees.
Co-reporter:Yilin Liu, Xiangqing Feng and Haifeng Du
Organic & Biomolecular Chemistry 2015 vol. 13(Issue 1) pp:125-132
Publication Date(Web):03 Oct 2014
DOI:10.1039/C4OB01087F
As an attractive class of non-biaryl atropisomeric compounds, C–N axially chiral anilides have received considerable attention, and several methods have been successfully developed for their synthesis. Pd-catalyzed asymmetric allylic amination was proved to be an effective approach for the chiral anilide synthesis, although only moderate enantioselectivity and relatively narrow substrate scope have been achieved in the previous work. Searching for highly efficient methods for the synthesis of axially chiral anilides is therefore of great interest in synthetic and pharmaceutical chemistry. In this paper, a palladium-catalyzed asymmetric allylic substitution of ortho-substituted anilides using phosphorus amidite–olefin ligands was successfully achieved to afford a variety of axially chiral anilides in high yields with up to 84% ee. The absolute configurations of chiral anilides were also determined from X-ray and CD spectra.
Co-reporter:Dr. Yongbing Liu;Lianrui Hu;Dr. Hui Chen;Dr. Haifeng Du
Chemistry - A European Journal 2015 Volume 21( Issue 8) pp:3495-3501
Publication Date(Web):
DOI:10.1002/chem.201405388
Abstract
The stereoselective hydrogenation of alkynes to alkenes is an extremely useful transformation in synthetic chemistry. Despite numerous reports for the synthesis of Z-alkenes, the hydrogenation of alkynes to give E-alkenes is still not well resolved. In particular, selective preparation of both Z- and E-alkenes by the same catalytic hydrogenation system using molecular H2 has rarely been reported. In this paper, a novel strategy of using simple alkenes as promoters for the HB(C6F5)2-catalyzed metal-free hydrogenation of alkynes was adopted. Significantly, both Z- and E-alkenes can be furnished by hydrogenation with molecular H2 in high yields with excellent stereoselectivities. Further experimental and theoretical mechanistic studies suggest that interactions between H and F atoms of the alkene promoter, borane intermediate, and H2 play an essential role in promoting the hydrogenolysis reaction.
Co-reporter:Zhenhua Zhang ;Dr. Haifeng Du
Angewandte Chemie International Edition 2015 Volume 54( Issue 2) pp:623-626
Publication Date(Web):
DOI:10.1002/anie.201409471
Abstract
A wide range of 2,3-disubstituted quinoxalines have been successfully hydrogenated with H2 using borane catalysts to produce the desired tetrahydroquinoxalines in 80–99 % yields with excellent cis selectivity. Significantly, the asymmetric reaction employing chiral borane catalysts generated by the in situ hydroboration of chiral dienes with HB(C6F5)2 under mild reaction conditions has also been achieved with up to 96 % ee, and represents the first catalytic asymmetric system to furnish optically active cis-2,3-disubstituted 1,2,3,4-tetrahydroquinoxalines.
Co-reporter:Zhenhua Zhang ;Dr. Haifeng Du
Angewandte Chemie 2015 Volume 127( Issue 2) pp:633-636
Publication Date(Web):
DOI:10.1002/ange.201409471
Abstract
A wide range of 2,3-disubstituted quinoxalines have been successfully hydrogenated with H2 using borane catalysts to produce the desired tetrahydroquinoxalines in 80–99 % yields with excellent cis selectivity. Significantly, the asymmetric reaction employing chiral borane catalysts generated by the in situ hydroboration of chiral dienes with HB(C6F5)2 under mild reaction conditions has also been achieved with up to 96 % ee, and represents the first catalytic asymmetric system to furnish optically active cis-2,3-disubstituted 1,2,3,4-tetrahydroquinoxalines.
Co-reporter:Simin Wei
Journal of the American Chemical Society 2014 Volume 136(Issue 35) pp:12261-12264
Publication Date(Web):August 18, 2014
DOI:10.1021/ja507536n
Using a simple combination of tri-tert-butylphosphine and chiral borane generated in situ by the hydroboration of chiral diene with HB(C6F5)2 as a frustrated Lewis pair catalyst, a highly enantioselective metal-free hydrogenation of silyl enol ethers was successfully realized to furnish a variety of optically active secondary alcohols in 93–99% yields with 88->99% ee’s.
Co-reporter:Minyang Zhuang and Haifeng Du
Organic & Biomolecular Chemistry 2014 vol. 12(Issue 26) pp:4590-4593
Publication Date(Web):24 Apr 2014
DOI:10.1039/C4OB00526K
This paper describes an enantioselective intermolecular allylic amination catalyzed by a chiral Brønsted acid via a possible chiral contact ion pair intermediate. A variety of symmetrical or unsymmetrical allylic alcohols can be smoothly aminated to afford the desired products in moderate to high yields with good enantioselectivities and/or regioselectivities.
Co-reporter:Xiangqing Feng, Yanzhao Nie, Lanqiong Zhang, Jing Yang, Haifeng Du
Tetrahedron Letters 2014 Volume 55(Issue 33) pp:4581-4584
Publication Date(Web):13 August 2014
DOI:10.1016/j.tetlet.2014.06.074
A rhodium-catalyzed asymmetric 1,2-addition of arylboronic acids to isatins with chiral sulfur–alkene hybrid ligands was achieved, and a variety of 3-aryl-3-hydroxy-2-oxindoles were obtained in moderate to good yields with up to 85% ee and a biologically active compound was synthesized with this strategy.A rhodium-catalyzed asymmetric 1,2-additions of arylboronic acids to isatins with chiral sulfur–alkene hybrid ligands was achieved, and a variety of 3-aryl-3-hydroxy-2-oxindoles were obtained in moderate to good yields with up to 85% ee. And a biologically active compound was synthesized with this strategy.
Co-reporter:Xiangqing Feng, Haifeng Du
Tetrahedron Letters 2014 Volume 55(Issue 51) pp:6959-6964
Publication Date(Web):17 December 2014
DOI:10.1016/j.tetlet.2014.10.138
The activation of H2 for the catalytic hydrogenation of unsaturated compounds is one of the most useful reactions in both academia and chemical industry, which has long been predominated by the transition-metal catalysis. However, metal-free hydrogen activation represents a formidable challenge, and has been less developed. The recent emerging chemistry of frustrated Lewis pairs (FLPs) with a combination of sterically encumbered Lewis acids and Lewis bases provides a promising approach for metal-free hydrogenation due to their amazing abilities for the challenging H2 activation. In the past several years, the hydrogenation of a wide range of unsaturated compounds using FLP catalysts has been successfully developed. Despite these advances, the corresponding asymmetric hydrogenation is just in its start-up step. Similar to the mode of HH bond activation, SiH bond can also be activated by FLPs for the hydrosilylation of ketones and imines. But its asymmetric version is also not well-solved. This Letter will outline the recent important progress of metal-free catalytic asymmetric hydrogenation and hydrosilylation using FLP catalysts.The metal-free hydrogenation utilizing molecular hydrogen is a challenging and unsolved problem. The development of frustrated Lewis pairs (FLPs) provides a novel access to the metal-free homogeneous hydrogenation. In the past several years, the hydrogenation of a wide range of unsaturated compounds using FLP catalysts has been successfully developed, and the asymmetric hydrogenation has also witnessed great progress. Similarly, SiH bond can also be activated by FLPs for hydrosilylation of ketones and imines. This Letter will outline the present progress of metal-free catalytic asymmetric hydrogenation and hydrosilylation using FLP catalysts.Figure optionsDownload full-size imageDownload as PowerPoint slide
Co-reporter:Yongbing Liu
Journal of the American Chemical Society 2013 Volume 135(Issue 35) pp:12968-12971
Publication Date(Web):August 14, 2013
DOI:10.1021/ja406761j
A metal-free direct hydrogenation of pyridines was successfully realized by using homogeneous borane catalysts generated from alkenes and HB(C6F5)2 via in situ hydroboration. The reaction affords a broad range of piperidines in high yields with excellent cis stereoselectivities.
Co-reporter:Yilin Liu and Haifeng Du
Organic Letters 2013 Volume 15(Issue 4) pp:740-743
Publication Date(Web):February 7, 2013
DOI:10.1021/ol3032736
A palladium-catalyzed asymmetric allylic alkylation of 3-substituted indoles using P/olefin ligands was successfully achieved to afford a variety of indolenines containing a quaternary carbon stereocenter in high yields with up to 87% ee. Significantly, this reaction provides a concise access to a stereoisomer of the natural product Angelicastigmin.
Co-reporter:Minyang Zhuang and Haifeng Du
Organic & Biomolecular Chemistry 2013 vol. 11(Issue 9) pp:1460-1462
Publication Date(Web):16 Jan 2013
DOI:10.1039/C3OB27285K
This paper describes a chiral Brønsted acid catalyzed asymmetric 1,2-rearrangement of racemic epoxides via a hydrogen-shift process for the synthesis of chiral aldehydes, and, followed by a reduction, a variety of optically active alcohols can be furnished in moderate yields with up to 50% ee. Especially, a facile one-pot synthesis of chiral alcohols directly from simple alkenes by a sequential epoxidation, rearrangement, and reduction has also been realized.
Co-reporter:Zhaoqun Liu, Xiangqing Feng, and Haifeng Du
Organic Letters 2012 Volume 14(Issue 12) pp:3154-3157
Publication Date(Web):June 6, 2012
DOI:10.1021/ol301248d
A simple chiral sulfur−alkene hybrid ligand has proven to be highly effective for rhodium-catalyzed formal cycloaddition reactions of racemic butadiene monoxide and imines to furnish spirooxindole oxazolidines or 1,3-oxazolidines with high yields and stereoselectivities. A possible dynamic kinetic resolution as well as a kinetic resolution is considered to be involved in this catalytic process.
Co-reporter:Xiangqing Feng, Yanzhao Nie, Jing Yang, and Haifeng Du
Organic Letters 2012 Volume 14(Issue 2) pp:624-627
Publication Date(Web):January 10, 2012
DOI:10.1021/ol203238j
This paper describes a Rh(I)-catalyzed highly efficient and enantioselective 1,2-addition of arylboronic acids to α-diketones with the use of a simple sulfur–alkene hybrid ligand. With as low as a 0.1 mol % catalyst loading, a variety of optically active α-hydroxyketones can be furnished in high yields with excellent ee’s.
Co-reporter:Dr. Xiangqing Feng ;Dr. Haifeng Du
Asian Journal of Organic Chemistry 2012 Volume 1( Issue 3) pp:204-213
Publication Date(Web):
DOI:10.1002/ajoc.201200069
Abstract
Chiral olefins have recently emerged as among the most promising ligands for asymmetric catalysis. In the past nearly ten years, significant advances have been made in developing ligands, including diene and heteroatom/olefin hybrid ligands, and exploring their applications in challenging metal-catalyzed asymmetric reactions. Especially in some cases, chiral olefin ligands have an obvious advantage over some conventional chiral ligands in terms of activity and selectivity. Besides the most often studied rigidly bicyclic dienes, readily accessible chiral-chain dienes with flexible frameworks and two terminal olefins as coordinating groups have also proven to be effective for achieving high levels of enantioselectivity in transition-metal-catalyzed asymmetric reactions. Moreover, the terminal olefins can be combined with phosphorus atoms on suitable chiral skeletons for the development of novel phosphorus/olefin (P/olefin) hybrid ligands, which have high activity and selectivity in Pd-catalyzed asymmetric transformations. Significantly, sulfur/olefin (S/olefin) hybrid ligands have been successfully explored and applied in a range of Rh-catalyzed asymmetric transformations. This Focus Review will mainly discuss the synthesis of recently developed chiral-chain diene ligands, phosphorus/terminal-olefin hybrid ligands, and sulfur/olefin hybrid ligands, and their application in asymmetric catalysis.
Co-reporter:Yilin Liu, Ziping Cao, and Haifeng Du
The Journal of Organic Chemistry 2012 Volume 77(Issue 9) pp:4479-4483
Publication Date(Web):April 11, 2012
DOI:10.1021/jo300539y
A palladium-catalyzed highly enantioselective allylic alkylation of pyrroles and 4,7-dihydroindoles has been successfully developed with the use of chiral alkene-phosphine hybrid ligands to furnish the desired products in high yields with excellent ee’s. It is noteworthy that alkene–phosphine ligands are much more effective than some other types of chiral ligands in this catalytic system.
Co-reporter:Xiangqing Feng, Beibei Wei, Jing Yang and Haifeng Du
Organic & Biomolecular Chemistry 2011 vol. 9(Issue 17) pp:5927-5929
Publication Date(Web):05 Jul 2011
DOI:10.1039/C1OB05971H
One novel type of chiral olefin/sulfinimide hybrid ligands has been developed through a simple one-step condensation of α,β-unsaturated ketones with tert-butanesulfinamide and utilized successfully for rhodium-catalyzed asymmetric conjugated additions to furnish the desired adducts in high yields with excellent ee's.
Co-reporter:Zhaoqun Liu, Ziping Cao and Haifeng Du
Organic & Biomolecular Chemistry 2011 vol. 9(Issue 15) pp:5369-5372
Publication Date(Web):14 Jun 2011
DOI:10.1039/C1OB05803G
This paper describes the development of a type of novel P–olefin hybrid ligand by the incorporation of terminal olefins onto phosphorus amidite ligands for palladium-catalyzed asymmetric allylic alkylations of indoles and substitutions with amines to give the desired products in 70–97% yield with 91–98% ee.
Co-reporter:Ziping Cao, Zhaoqun Liu, Yilin Liu, and Haifeng Du
The Journal of Organic Chemistry 2011 Volume 76(Issue 15) pp:6401-6406
Publication Date(Web):June 13, 2011
DOI:10.1021/jo200925q
Palladium-catalyzed asymmetric allylic etherizations with a variety of oximes as nucleophiles utilizing a chiral alkene-phosphine hybrid ligand have been successfully achieved for the first time to afford the optical active oxime ethers in high yields with good to excellent enantioselectivities.
Co-reporter:Yazhou Wang, Xichao Hu, and Haifeng Du
Organic Letters 2010 Volume 12(Issue 23) pp:5482-5485
Publication Date(Web):November 1, 2010
DOI:10.1021/ol1023536
A variety of readily accessible vicinal-diamine-based chiral chain dienes were successfully synthesized and utilized as steering ligands for rhodium-catalyzed conjugated additions of organoboronic acids to α,β-unsaturated carbonyl compounds to afford the desired adducts in good to excellent yields and ee’s.
Co-reporter:Ziping Cao and Haifeng Du
Organic Letters 2010 Volume 12(Issue 11) pp:2602-2605
Publication Date(Web):May 12, 2010
DOI:10.1021/ol1008087
A variety of binaphthyl-based chiral dienes were synthesized and utilized as steering ligands for the enantioselective arylation of N,N-dimethylsulfamoyl-protected aldimines with arylboronic acids, and moderate to good yields and up to 84% ee were achieved.
Co-reporter:Zhaoqun Liu and Haifeng Du
Organic Letters 2010 Volume 12(Issue 13) pp:3054-3057
Publication Date(Web):June 10, 2010
DOI:10.1021/ol101069y
A variety of novel chiral terminal-alkene−phosphine hybrid ligands were successfully developed from diethyl l-tartrate for palladium-catalyzed asymmetric allylic alkylations, etherifications, and amination to give the desired products in excellent yields and ee’s.
Co-reporter:Xichao Hu;Ziping Cao;Zhaoqun Liu;Yazhou Wang
Advanced Synthesis & Catalysis 2010 Volume 352( Issue 4) pp:651-655
Publication Date(Web):
DOI:10.1002/adsc.200900693
Abstract
A variety of acyclic chiral dienes were synthesized in a single step via palladium(0)-catalyzed asymmetric allylic and homoallylic CH diamination of terminal olefins. The applications of such simple dienes as steering ligands for rhodium(I)-catalyzed asymmetric 1,4-additions afforded the corresponding adducts in excellent yields and up to 85% ee.
Co-reporter:Xichao Hu, Minyang Zhuang, Ziping Cao and Haifeng Du
Organic Letters 2009 Volume 11(Issue 20) pp:4744-4747
Publication Date(Web):September 17, 2009
DOI:10.1021/ol901949n
This paper describes that a simple and readily available chain diene (3R,4R)-hexa-1,5-diene-3,4-diol (L1) was successfully utilized as a novel steering ligand for Rh(I)-catalyzed asymmetric conjugated additions. Encouraging yields and ee’s have been achieved, which may provide a new and practical direction for designing chiral diene ligands in the future.
Co-reporter:Yongbing Liu
Journal of the American Chemical Society () pp:
Publication Date(Web):April 22, 2013
DOI:10.1021/ja4025808
This paper describes a highly enantioselective metal-free hydrogenation of imines using chiral dienes as “ligands” for the generation of catalysts with HB(C6F5)2 by hydroboration in situ to furnish a variety of chiral amines with up to 89% ee, which provides a practical strategy for the development of novel chiral frustrated Lewis pairs for asymmetric hydrogenation.
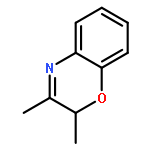
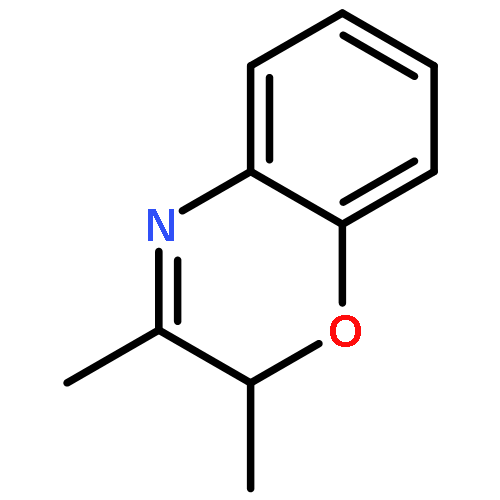
Co-reporter:Simin Wei, Xiangqing Feng and Haifeng Du
Organic & Biomolecular Chemistry 2016 - vol. 14(Issue 34) pp:NaN8029-8029
Publication Date(Web):2016/08/01
DOI:10.1039/C6OB01556E
A metal-free hydrogenation of 3-substituted 2H-1,4-benzoxazines has been successfully realized with 2.5 mol% of B(C6F5)3 as a catalyst to furnish a variety of 3,4-dihydro-2H-1,4-benzoxazines in 93–99% yields. Up to 42% ee was also achieved for the asymmetric hydrogenation with the use of a chiral diene and HB(C6F5)2.
Co-reporter:Wei Wang, Xiangqing Feng and Haifeng Du
Organic & Biomolecular Chemistry 2016 - vol. 14(Issue 28) pp:NaN6686-6686
Publication Date(Web):2016/06/16
DOI:10.1039/C6OB01172A
Metal-free hydrogenation of 2,7-disubstituted 1,8-naphthyridines was successfully realized for the first time using in situ generated borane catalysts under mild conditions to furnish 1,2,3,4-tetrahydro-1,8-naphthyridine derivatives in 83–98% yields. Significantly, up to 74% ee was achieved for the corresponding asymmetric hydrogenation reactions.
Co-reporter:Xiangqing Feng, Beibei Wei, Jing Yang and Haifeng Du
Organic & Biomolecular Chemistry 2011 - vol. 9(Issue 17) pp:NaN5929-5929
Publication Date(Web):2011/07/05
DOI:10.1039/C1OB05971H
One novel type of chiral olefin/sulfinimide hybrid ligands has been developed through a simple one-step condensation of α,β-unsaturated ketones with tert-butanesulfinamide and utilized successfully for rhodium-catalyzed asymmetric conjugated additions to furnish the desired adducts in high yields with excellent ee's.
Co-reporter:Zhaoqun Liu, Ziping Cao and Haifeng Du
Organic & Biomolecular Chemistry 2011 - vol. 9(Issue 15) pp:NaN5372-5372
Publication Date(Web):2011/06/14
DOI:10.1039/C1OB05803G
This paper describes the development of a type of novel P–olefin hybrid ligand by the incorporation of terminal olefins onto phosphorus amidite ligands for palladium-catalyzed asymmetric allylic alkylations of indoles and substitutions with amines to give the desired products in 70–97% yield with 91–98% ee.
Co-reporter:Minyang Zhuang and Haifeng Du
Organic & Biomolecular Chemistry 2013 - vol. 11(Issue 9) pp:NaN1462-1462
Publication Date(Web):2013/01/16
DOI:10.1039/C3OB27285K
This paper describes a chiral Brønsted acid catalyzed asymmetric 1,2-rearrangement of racemic epoxides via a hydrogen-shift process for the synthesis of chiral aldehydes, and, followed by a reduction, a variety of optically active alcohols can be furnished in moderate yields with up to 50% ee. Especially, a facile one-pot synthesis of chiral alcohols directly from simple alkenes by a sequential epoxidation, rearrangement, and reduction has also been realized.
Co-reporter:Gen Li, Yongbing Liu and Haifeng Du
Organic & Biomolecular Chemistry 2015 - vol. 13(Issue 10) pp:NaN2878-2878
Publication Date(Web):2015/01/19
DOI:10.1039/C5OB00009B
A catalytic metal-free hydrogenation of naphthylamines using B(C6F5)3 as a catalyst was successfully achieved under mild conditions for the first time to furnish a variety of tetrahydronaphthylamines in 88–99% yields.
Co-reporter:Xiaxia Zhu and Haifeng Du
Organic & Biomolecular Chemistry 2015 - vol. 13(Issue 4) pp:NaN1016-1016
Publication Date(Web):2014/11/28
DOI:10.1039/C4OB02419B
An enantioselective hydrosilylation of imines was successfully achieved using a chiral borane catalyst generated by the in situ hydroboration of a binaphthyl-based chiral diene with Piers’ borane HB(C6F5)2 to furnish a variety of optically active amines in 70% to >99% yields and 44–82% ees.
Co-reporter:Minyang Zhuang and Haifeng Du
Organic & Biomolecular Chemistry 2014 - vol. 12(Issue 26) pp:NaN4593-4593
Publication Date(Web):2014/04/24
DOI:10.1039/C4OB00526K
This paper describes an enantioselective intermolecular allylic amination catalyzed by a chiral Brønsted acid via a possible chiral contact ion pair intermediate. A variety of symmetrical or unsymmetrical allylic alcohols can be smoothly aminated to afford the desired products in moderate to high yields with good enantioselectivities and/or regioselectivities.
Co-reporter:Gen Li, Xiangqing Feng and Haifeng Du
Organic & Biomolecular Chemistry 2015 - vol. 13(Issue 20) pp:NaN5830-5830
Publication Date(Web):2015/04/16
DOI:10.1039/C5OB00663E
Isatins and their derivatives are important functional moities and building blocks in pharmaceutical and synthetic chemistry. Numerous enantioselective transformations at the C-3 carbonyl group have been well developed. However, the asymmetric substitution reaction with isatins and their derivatives as nucleophiles based on the free N–H groups has been less studied due to the relatively weaker nucleophilicity resulting from the two electron-withdrawing carbonyl groups. In this paper, a palladium-catalyzed asymmetric allylic amination of racemic butadiene monoxide with isatin derivatives using a chiral phosphoramidite olefin hybrid ligand has been successfully developed under mild conditions. A variety of chiral amino alcohols were afforded in 55–87% yields with 10/1->20/1 regioselectivity ratios and 80–97% ees.
Co-reporter:Yilin Liu, Xiangqing Feng and Haifeng Du
Organic & Biomolecular Chemistry 2015 - vol. 13(Issue 1) pp:NaN132-132
Publication Date(Web):2014/10/03
DOI:10.1039/C4OB01087F
As an attractive class of non-biaryl atropisomeric compounds, C–N axially chiral anilides have received considerable attention, and several methods have been successfully developed for their synthesis. Pd-catalyzed asymmetric allylic amination was proved to be an effective approach for the chiral anilide synthesis, although only moderate enantioselectivity and relatively narrow substrate scope have been achieved in the previous work. Searching for highly efficient methods for the synthesis of axially chiral anilides is therefore of great interest in synthetic and pharmaceutical chemistry. In this paper, a palladium-catalyzed asymmetric allylic substitution of ortho-substituted anilides using phosphorus amidite–olefin ligands was successfully achieved to afford a variety of axially chiral anilides in high yields with up to 84% ee. The absolute configurations of chiral anilides were also determined from X-ray and CD spectra.
Co-reporter:Wei Wang, Wei Meng and Haifeng Du
Dalton Transactions 2016 - vol. 45(Issue 14) pp:NaN5948-5948
Publication Date(Web):2015/12/03
DOI:10.1039/C5DT03872C
This paper describes the first metal-free hydrogenation of 3,6-diarylpyridazines, which was successfully realized using B(C6F5)3 as a catalyst. A variety of 1,4,5,6-tetrahydropyridazine derivatives were furnished in 85–95% yields.

![Pyridazine, 3-[(4-methoxyphenyl)methyl]-6-phenyl-](http://img.cochemist.com/ccimg/927400/927396-11-0.png)
![Pyridazine, 3-[(4-methoxyphenyl)methyl]-6-phenyl-](http://img.cochemist.com/ccimg/927400/927396-11-0_b.png)
![N-[1-(2-METHYLPHENYL)ETHYL]ANILINE](http://img.cochemist.com/ccimg/791900/791808-29-2.png)
![N-[1-(2-METHYLPHENYL)ETHYL]ANILINE](http://img.cochemist.com/ccimg/791900/791808-29-2_b.png)