Co-reporter:Mohammed A. H. Alamiry, Andrew C. Benniston, Jerry Hagon, Thomas P. L. Winstanley, Helge Lemmetyinen and Nikolai V. Tkachenko
RSC Advances 2012 vol. 2(Issue 11) pp:4944-4950
Publication Date(Web):27 Mar 2012
DOI:10.1039/C2RA20219K
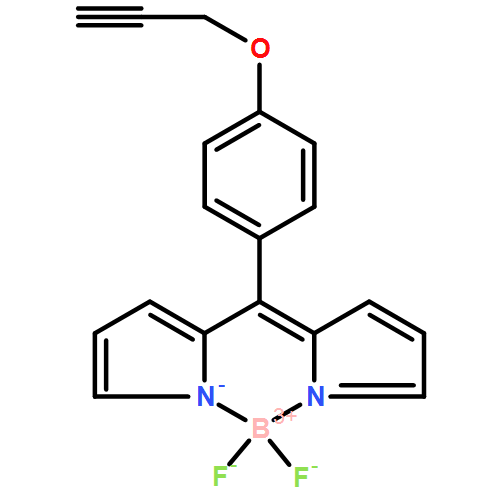
A small series of partially alkylated Bodipy dyes were prepared containing Fn-aryl (n = 1,2,3,5) groups at the meso position. The effect of increasing the number of fluorines, and their position in the aryl ring, on the electrochemical and photophysical properties of the dyes is discussed. The highly electron withdrawing pentafluoroaryl group makes reduction of the Bodipy segment especially easy when compared to the basic phenylene derivative. High quantum yields of fluorescence in toluene solution are seen for derivatives with fluorine(s) substituted in the ortho position of the meso aryl group. This effect is also mirrored in the fluorescence lifetimes for the molecules. Pressure dependent fluorescence measurements carried out reveal subtle differences in the behaviour for the series of Bodipy derivatives.


![Dibenzo[e,g][1,4]dioxocin-3,10-diamine, 6,7-dihydro-](http://img.cochemist.com/ccimg/563600/563539-69-5.png)
![Dibenzo[e,g][1,4]dioxocin-3,10-diamine, 6,7-dihydro-](http://img.cochemist.com/ccimg/563600/563539-69-5_b.png)
![9,12-Dioxa-2,3-diazatricyclo[11.3.1.14,8]octadeca-1(17),2,4,6,8(18),13,
15-heptaene](http://img.cochemist.com/ccimg/563600/563539-62-8.png)
![9,12-Dioxa-2,3-diazatricyclo[11.3.1.14,8]octadeca-1(17),2,4,6,8(18),13,
15-heptaene](http://img.cochemist.com/ccimg/563600/563539-62-8_b.png)
![Dibenzo[q,s][1,4,7,10,13,16]hexaoxacycloeicosin, 6,7,9,10,12,13,15,16,18,19-decahydro-](/data/chemimg/3724000/41051-91-6.png)
![Dibenzo[q,s][1,4,7,10,13,16]hexaoxacycloeicosin, 6,7,9,10,12,13,15,16,18,19-decahydro-](/data/chemimg/3724000/41051-91-6_b.png)