Co-reporter:On Ying Yuen, Chau Ming So and Fuk Yee Kwong
RSC Advances 2016 vol. 6(Issue 33) pp:27584-27589
Publication Date(Web):15 Mar 2016
DOI:10.1039/C6RA03188A
A palladium(II)-catalyzed oxidative Mizoroki–Heck reaction of arylsulfonyl hydrazides with alkenes was developed employing atmospheric air as the sole oxidant in an open-vessel manner. By using palladium(II) acetate associating with inexpensive, air-stable and moisture stable pyridine ligand L9 as the catalyst system, the efficiency of the reaction could be significantly enhanced. A wide range of arylsulfonyl hydrazides underwent the oxidative Mizoroki–Heck reaction with alkenes smoothly. Good-to-excellent product yields and excellent regio- and stereoselectivity were achieved. Functional groups such as halo, ester etc. were well-tolerated under these optimized reaction conditions.
Co-reporter:Wai Chung Fu, Chau Ming So, Wing Kin Chow, On Ying Yuen, and Fuk Yee Kwong
Organic Letters 2015 Volume 17(Issue 18) pp:4612-4615
Publication Date(Web):September 9, 2015
DOI:10.1021/acs.orglett.5b02344
The rational design of a phosphine ligand for the reductive elimination-demanding Pd-catalyzed mono-α-arylation of acetone is demonstrated and reported. The catalyst is tolerant of previously proven challenging electron-deficient aryl chlorides and provides excellent product yields with down to 0.1 mol % Pd. Preliminary investigations suggest that the rate-limiting step for the proposed system is the oxidative addition of aryl chlorides, in which it contradicts previous findings regarding the α-arylation of acetone with aryl halides.

![Methanone, [4-(1-heptynyl)phenyl]phenyl-](http://img.cochemist.com/ccimg/882500/882429-00-7.png)
![Methanone, [4-(1-heptynyl)phenyl]phenyl-](http://img.cochemist.com/ccimg/882500/882429-00-7_b.png)
![Quinoline, 6-[(4-methoxyphenyl)ethynyl]-](http://img.cochemist.com/ccimg/857600/857507-27-8.png)
![Quinoline, 6-[(4-methoxyphenyl)ethynyl]-](http://img.cochemist.com/ccimg/857600/857507-27-8_b.png)
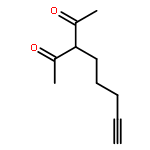
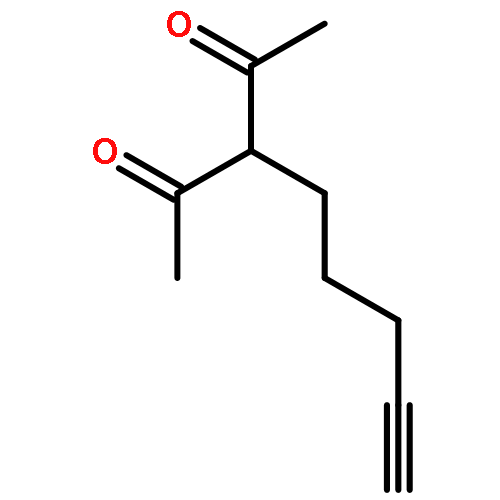
![Benzenamine, N-[4-(1,1-dimethylethyl)phenyl]-2,6-dimethyl-](http://img.cochemist.com/ccimg/628400/628333-88-0.png)
![Benzenamine, N-[4-(1,1-dimethylethyl)phenyl]-2,6-dimethyl-](http://img.cochemist.com/ccimg/628400/628333-88-0_b.png)
![1-Butanamine, N,N-bis[(diphenylphosphino)methyl]-](http://img.cochemist.com/ccimg/645000/644998-09-4.png)
![1-Butanamine, N,N-bis[(diphenylphosphino)methyl]-](http://img.cochemist.com/ccimg/645000/644998-09-4_b.png)








![2H-1-Benzopyran-2-one, 6-methyl-4-[(methylsulfonyl)oxy]-](http://img.cochemist.com/ccimg/820300/820209-48-1.png)
![2H-1-Benzopyran-2-one, 6-methyl-4-[(methylsulfonyl)oxy]-](http://img.cochemist.com/ccimg/820300/820209-48-1_b.png)